Abstract
Background
Weight loss in patients with cancer is caused by cancer cachexia and chemotherapy-induced nausea and vomiting (CINV). Recent developments in antiemetic drugs have substantially improved CINV, but nutritional intervention did not improve body weight. This study aimed to investigate the effects of nutrition intervention with appropriate antiemetic treatment in patients with non-small-cell lung cancer during chemotherapy.
Methods
Patients received individualized nutrition counseling by a registered dietitian and were provided with oral supplements for 90 days. Body weight and other parameters were measured at baseline and after 90-day intervention. To evaluate this nutrition intervention, patients were also retrospectively set as control, and then body weight change was compared with inverse probability of treatment weights (IPTW) analysis.
Results
Ten patients received individualized nutrition counseling and were provided with oral supplements for 90 days. Of them, 7 patients consumed nutritional supplements, and the mean intake was 130 kcal/day. After 90-day intervention, the patients did not show significant weight and BMI loss during the course of cytotoxic chemotherapy. A total of 38 patients were retrospectively enrolled as controls. The number of the patients who gain the body weight after 90 days in the study cohort was significantly larger than that in the retrospective controls with the IPTW analysis (Odds Ratio (OR) = 8.4; 95% Confidence Interval (CI): 1.6–42; P = 0.01).
Conclusion
Early intensive nutrition intervention with appropriate antiemetic treatment prevents weight loss. Nutrition interventions might be also beneficial for quality of life, treatment response and survival.
Keywords: nausea, non-small-cell lung cancer, nutrition intervention, vomiting, weight loss
Weight loss is common among patients with advanced cancer and contributes to poor treatment response, deterioration in quality of life (QOL) and decreased survival.1 There are two types of weight loss in patients with cancer: cancer cachexia and chemotherapy-induced weight loss.
Cancer cachexia is particularly common in lung, gastric, pancreatic and prostate cancers.2 The pathophysiology of cachexia is multifactorial and is still not well-understood. It is proposed to be the result of tumor-host interactions.3 The cachexia guideline expert group identified cancer cachexia as a continuum of three stage of clinical relevance: pre-cachexia, cachexia and refractory cachexia.4 Refractory cachexia represents a stage where reversal of weight loss seems no longer possible. In this stage, the burdens and risks of artificial nutritional support likely outweigh expected benefit. Early, namely the stage of pre-cachexia intervention is essential.4
The major causes of chemotherapy-induced weight loss are adverse events such as nausea and vomiting. Chemotherapy-induced nausea and vomiting (CINV) is the most common and intolerable adverse event of cytotoxic chemotherapy. It causes reduced oral intake5 and has negative impacts on QOL.6, 7 To prevent chemotherapy-induced weight loss, several nutrition interventions such as dietary counseling (DC) and high-energy oral nutritional supplement (ONS) for patients with advanced cancer have been undertaken. However, these interventions have shown no improvement in nutritional parameters8–11 owing to poor compliance to the nutritional intervention and poor treatment outcomes of CINV.8 In brief, uncontrollable CINV reduced the positive effects of nutritional interventions with DC and ONS.
Fortunately, recent advances in CINV treatment for the past 10 years, particularly the development of new-generation antiemetic drugs, has considerably improved outcomes. The efficacy of aprepitant for CINV was confirmed in clinical trials globally12–14 including in Japan.15 It was approved by the United States Food and Drug Administration in 2003 and by the Ministry of Health, Labor and Welfare in Japan in 2009. Major clinical practice guidelines on antiemetics classify cisplatin-based chemotherapies as highly emetogenic chemotherapies (HEC) and strongly recommend aprepitant-containing 3-drug regimens, including a 5-hydroxytryptamine 3 receptor antagonist (5-HT3RA) and dexamethasone.16–19 Since then, acute- and delayed-phase CINV has been less severe, and the QOL of patients with cancer has improved.6, 7
However, weight loss still occurs frequently despite antiemetic treatments in patients with lung cancer during cytotoxic chemotherapy. Weight loss is generally caused by reduced oral intake and is a serious problem among patients with cancer.5 Most previous studies on nutrition interventions with DC and/or ONS8–11 have been undertaken before new guidelines on antiemetics have been established.16–19 This study aimed to investigate the effect of early nutrition intervention on body weight during the course of chemotherapy with appropriate antiemetic treatments in patients with lung cancer. We hypothesized that intensive nutrition intervention may prevent chemotherapy-induced weight loss based on the appropriate antiemetic treatments.
MATERIALS AND METHODS
Patients
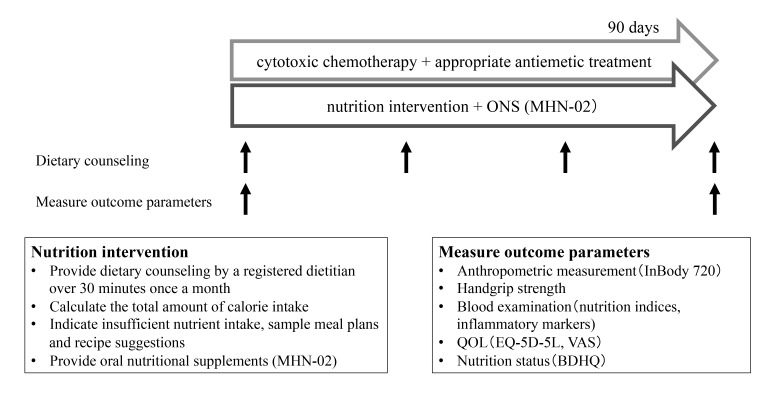
This study is registered in the UMIN registry system (No. 000030785). Consecutive patients with histologically proven inoperable or recurrent non-small-cell lung cancer between August 2017 and May 2018 in Tottori University Hospital, Japan, who were scheduled for the first line cytotoxic chemotherapy were eligible for inclusion. Patients received individualized nutrition counseling by a registered dietitian and were provided with ONS for 90 days. The duration was set based on standard period of first line chemotherapy. To evaluate the nutrition intervention, we also retrospectively enrolled patients as control. The following outcome parameters were measured at baseline and 90 days after commencing chemotherapy.
The following outcome parameters were measured at baseline and 90 days after commencing chemotherapy (Fig. 1).
Fig. 1.
Flow of research process. Patients received nutrition intervention for 90 days during cytotoxic chemotherapy. Body weight and other parameters were measured at baseline and after 90-day intervention. BDHQ, brief-type self-administered diet history questionnaire; EQ-5D-5L, EuroQol-5 Dimensions, 5Levels; ONS, oral nutritional supplement; QOL, quality of life; VAS, Visual Analogue Scale.
Retrospective controls
To compare between patients with and without early intensive nutrition intervention, we retrospectively reviewed the clinical data and body weight of 38 consecutive patients with inoperable or recurrent non-small-cell lung cancer who were given cytotoxic chemotherapy in Tottori University Hospital between April 2014 and March 2016. These patients were considered as the retrospective controls. They all received three or more cycles of cytotoxic chemotherapies with new recommended antiemetic drugs.
Nutrition intervention
Patients received individualized nutrition interventions in the form of intensive nutrition counseling by a registered dietitian once a month. They were held in the interview room for 30 minutes or more. A registered dietitian calculated the total amount of calorie intake through counseling and questionnaire (described below for details) and determined those with insufficient nutrient intake. To estimate total energy needs, physical activity and disease factors were included. Basal energy expenditure (BEE) was estimated using the Harris Benedict equation.20 To calculate total energy expenditure (TEE), we multiplied BEE by an activity factor of 1.4 by an injury factor of 1.2.21 Based on the actual calorie intake and requirements, a registered dietitian provided individual sample meal plans, recipe suggestions, and suggestions to minimize the side effects of the tumor pathology and therapy. They were recommended to take nutritional supplements to increase calorie intake. Attending doctors, dietitians, and nurses comprised the nutrition intervention team, and they conducted a nutrition meeting once monthly to discuss the patient’s nutrition status and other clinical information.
Nutritional supplement
MHN-02(MEIN, Meiji Dairies Corporation, Tokyo, Japan) was used as ONS in this study. In general, MHN-02 is a whey peptide with anti-inflammatory and antioxidant components such as vitamins C and E and enriched trace elements (i.e., zinc and selenium).22 Patients were allowed to consume as much as they wanted based on nutrition counseling, and the daily intakes were recorded in a diary.
Data collection
Anthropometric measurements
Body composition was taken by the same two trained investigators throughout the study and analyzed with bioelectrical impedance analysis with an InBody720 (InBody Japan Corp. Tokyo, Japan). This system uses an electrical current at different frequencies (5, 50, 250, 500, and 1000 kHz) to directly measure the amount of extracellular and intracellular water in the body. The patient stands with the soles in contact with the foot electrodes and grabs the hand electrodes. Data output included fat mass, skeletal muscle mass of the total body, arms and legs, and basal metabolic rate and was calculated by using the manufacturer’s algorithm. The muscle mass was converted into the skeletal muscle mass index (SMI) by dividing the weight by the height squared (kg/m2).
Handgrip strength
Handgrip strength was measured using a digital grip-strength meter (TKK5401; Takei Scientific Instruments Co, Ltd, Niigata City, Japan). Participants performed the tests twice in both hands in a standing position. The mean of measurements was used.
Blood examination
For biochemical testing, total protein, albumin, and hemoglobin were measured as nutrition indices. C-reactive protein and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) were measured as inflammatory markers. Standard blood laboratory parameters were analyzed using fresh samples in accordance with routine protocols at the hospital. Serum pro-inflammatory cytokines were measured using commercially available enzyme-linked immunosorbent assay kits (R&D systems, Inc. Minneapolis, MN).
Quality-of-life assessment
QOL was measured using EuroQol-5 Dimensions, 5 Levels (EQ-5D-5L). We used Japanese interim EQ-5D value set estimated with official crosswalk methodology as developed by the EuroQol Group, which is a validated measure of health-related QOL. It comprises a five-dimension health status classification system and a separate Visual Analogue Scale.23
Nutritional status
Patients answered a brief-type self-administered diet history questionnaire (BDHQ) to provide data for nutrition intake estimates.24, 25 The BDHQ comprised four pages with 75 questions that assessed dietary habits during the past 1 month: 55 questions were related to food consumption and 17 were on cooking and dietary behaviors. The daily intake estimates for foods, energy, and selected nutrients were calculated using an ad hoc computer algorithm developed for the BDHQ, which was based on the Standard Tables of Food Composition in Japan.
CINV
Nausea and vomiting that occurred within 14 days after commencing chemotherapy were defined as CINV and was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Event (version 4.0).
Statistical Analysis
Continuous data were expressed as mean ± standard deviation or median and interquartile range. Outcome parameters were compared between at baseline and after 90-day intervention using paired t-test. Multivariate logistic regression analysis was used to compare body weight and BMI changes between patients and retrospective controls. In addition, inverse probability of treatment weighting (IPTW)-adjusted analyses were performed to account for baseline differences.26 The propensity to undergo nutritional intervention in the patients versus retrospective controls was estimated using a logistic regression model based on age, sex, chemotherapy (platinum-based doublet chemotherapy or not), body height, and body weight. Propensity scores were then used to derive IPTW, with the inverse of the propensity score for patients of the study group and the inverse of 1 minus the propensity score for patients of the retrospective control group. Then, the generalized estimating equations were used to compare body weight change during 90 days in the two groups.
Statistical significance was determined as P < 0.05 with a two-sided test. All statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA) and SPSS Statistics 25 (IBM Japan Ltd., Tokyo, Japan).
Ethics
This study was reviewed and approved by the Ethics Committee of Tottori University (#1704B001) and was performed according to the Declaration of Helsinki.27 All patients provided written informed consent.
RESULTS
A total of 10 consecutive patients were included. They were recruited between August 2017 and May 2018 and followed up from August 2018 until trial ended. The cohort comprised 6 men and 4 women and the median age was 68.5 (range, 28–83) years. Table 1 shows the baseline characteristics of the patients. All patients received cytotoxic chemotherapy; eight patients received three cycles of chemotherapy, while two patients received only two cycles of chemotherapy because of disease progression. Eight patients received standard platinum-based doublet chemotherapies with recommended aprepitant-containing 3-drug antiemetic regimens, and the two patients received non-platinum single-agent chemotherapies with recommended 2-drug antiemetic regimens.
Table 1.
Patient characteristics of the study cohort
| Number | n = 10 | |
| Age (years), median (range) | 68.5 (28–83) | |
| Sex, n (%) | ||
| Male | 6 (60%) | |
| Female | 4 (40%) | |
| Smoking status, n (%) | ||
| Never | 2 (20%) | |
| Former or current | 8 (80%) | |
| Body weight (kg) | 56.4 ± 7.3 | |
| Body height (m) | 1.59 ± 0.1 | |
| Body mass index (kg/m2) | 22.4 ± 3.9 | |
| Histological type, n (%) | ||
| Adenocarcinoma | 7 (70%) | |
| Squamous cell carcinoma | 2 (20%) | |
| Adenosquamous carcinoma | 1 (10%) | |
| Mutation status, n (%) | ||
| EGFR | 1 (10%) | |
| EML4-ALK fusion | 1 (10%) | |
| ROS1 fusion | 1 (10%) | |
| Wild type or unknown | 7 (70%) | |
| Clinical stage, n (%) | ||
| IIIB or IV | 9 (90%) | |
| Recurrence | 1 (10%) | |
| Chemotherapy, n (%) | ||
| Platinum-based doublet chemotherapy | 8 (80%) | |
| Non-platinum chemotherapy | 2 (20%) | |
EGFR, epidermal growth factor receptor; EML4-ALK, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase fusion; n, number of cases (% total cases); ROS1 fusion, c-ros oncogene 1 receptor tyrosine kinase fusion. Data are presented as the mean ± SD.
All patients received 90-day nutrition intervention. Table 2 summarizes the outcome parameters of the patients. The mean body weight, BMI and QOL mildly increased; however, the increases were not statistically significant. The highest body weight gain was 6.3 kg, and the highest body weight loss was 2.0 kg because of disease progression. Handgrip strength showed significant decrease, while total SMI, arm SMI and leg SMI did not increase between baseline and after 90-day intervention.
Table 2.
Outcome parameters of the patients at baseline and after 90-day intervention
| Baseline | After 90-day intervention | P value | ||
| Anthropometric measurements | ||||
| Body weight (kg) | 56.4 ± 7.3 | 57.4 ± 7.1 | Ns | |
| BMI (kg m-2) | 22.4 ± 3.9 | 22.8 ± 4.3 | Ns | |
| SMI (kg m-2) | 6.4 ± 0.7 | 6.4 ± 0.6 | Ns | |
| Arm SMI (kg m-2) | 1.6 ± 0.3 | 1.6 ± 0.3 | Ns | |
| Leg SMI (kg m-2) | 4.7 ± 0.5 | 4.7 ± 0.3 | Ns | |
| Handgrip strength (kg) | 24.4 ± 6.7 | 21.7 ± 6.7 | 0.012 | |
| QOL assessment | ||||
| QOL (EQ-5D-5L), median (range) | 0.84 (0.49–1.00) | 0.89 (0.45–1.00) | Ns | |
| QOL (VAS) (mm) | 70.5 ± 22 | 71.5 ± 23 | Ns | |
| Blood examination | ||||
| CRP (mg dL-1) | 1.39 ± 1.8 | 1.10 ± 1.8 | Ns | |
| TNF-α (pg mL-1) | 26.3 ± 5.8 | 53.3 ± 40 | Ns | |
| IL-6 (pg mL-1) | 12.9 ± 6.7 | 9.9 ± 6.2 | Ns | |
| IL-1β (pg mL-1) | 29.9 ± 22 | 26.7 ± 21 | Ns | |
| Dietetic consumption* | ||||
| Kcal (Kcal/day) | 1618 ± 490 | 1652 ± 490 | Ns | |
| Proteins (g/day) | 61.8 ± 19 | 67.7 ± 28 | Ns | |
| CHO (g/day) | 226 ± 74 | 224 ± 92 | Ns | |
| Fat (g/day) | 46.0 ± 74 | 51.7 ± 24 | Ns | |
| n-3 fatty acid (g/day) | 2.0 ± 0.9 | 2.7 ± 1.4 | Ns | |
| α-linolenic acid (g/day) | 1.3 ± 0.8 | 1.6 ± 0.8 | Ns | |
| EPA (g/day) | 0.22 ± 0.13 | 0.42 ± 0.26 | 0.038 | |
| DHA (g/day) | 0.38 ± 0.18 | 0.56 ± 0.38 | Ns | |
*After 90-day intervention of dietetic consumption includes both food intake and nutritional supplement.
Data are presented as the mean ± SD. Statistical analysis was performed with paired t-test. P < 0.05 was considered statistically significant.
BMI, body mass index; CHO, carbohydrates; CRP, c-reactive protein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; EQ-5D-5L, EuroQol5 dimensions 5 levels; IL-1β, interleukin-1β; IL-6, interleukin-6; Kcal, kilocalories; Ns, not significant; QOL, quality of life; SMI, skeletal mass index; TNF-α, tumor necrosis factor; VAS, visual analogue scale.
The pro-inflammatory cytokines TNF-α, IL-6 and IL-1β play a central role in the pathogenesis of cancer-related body weight loss and cachexia.28, 29 Moreover, reduction in inflammation prevents body weight loss and cachexia.30, 31 In our study, the amounts of inflammatory markers such as CRP, TNF-α, IL-6 and IL-1β were higher than normal even at baseline, and they did not decrease after the 90-day intervention. The mean dietetic consumption of the participants at baseline was approximately 25% lower than the TEE, which was estimated using the Harris Benedict equation. TEE is affected by an activity factor and an injury factor. In addition, after the 90-day intervention, dietetic consumption did not show significant decrease although all patients received cytotoxic chemotherapies. Seven of the ten participants consumed nutritional supplements, and the mean intake was approximately 130 kcal/day. Eicosapentaenoic acid (EPA) intake significantly increased after the 90-day intervention. Meanwhile, there were no significant changes in statistics and dietetic consumption of total kilocalories, proteins, and fat after the 90-day intervention (Table 2).
In general, the body weight of patients with lung cancer tend to decrease during cytotoxic chemotherapy. In the present study, there were no remarkable decreases of body weight (Table 2). As for a comparison between patients with and without early intensive nutrition intervention, there were no significant characteristic differences between the study cohort and the retrospective controls. Moreover, the frequency of CINV was not significantly different between the two groups (Table 3). Meanwhile, compared with the retrospective control, the number of the patients who gain the body weight after 90 days in the study cohort was significantly larger than that in the retrospective controls with the IPTW analysis [Odds Ratio (OR) = 8.4; 95% Confidence Interval (CI): 1.6–42; P = 0.01] (Table 4)
Table 3.
Comparison of baseline characteristics between patients and retrospective controls
| Patients n = 10 | Retrospective controls n = 38 | P value | ||
| Age | 68.5 (28–83) | 73 (40–89) | Ns† | |
| Sex, n (%) | ||||
| Male | 6 (60%) | 30 (79%) | Ns‡ | |
| Female | 4 (40%) | 8 (21%) | ||
| Smoking status n (%) | ||||
| Never | 2 (20%) | 7 (18%) | Ns‡ | |
| Former or current | 8 (80%) | 31 (82%) | ||
| Histological type n (%) | ||||
| Adenocarcinoma | 7 (70%) | 29 (76%) | Ns‡ | |
| Squamous cell carcinoma | 2 (20%) | 9 (24%) | ||
| Adenosquamous carcinoma | 1 (10%) | 0 | ||
| Best response of chemotherapy n (%) | ||||
| PR or SD | 8 (80%) | 35 (92% | Ns‡ | |
| PD | 2 (20%) | 3 (8%) | ||
| CINV | ||||
| Nausea* | ||||
| All grade | 8 (80%) | 22 (58%) | Ns‡ | |
| Grade 3/4 | 1 (10%) | 2 (5%) | ||
| Vomiting* | ||||
| All grade | 2 (20%) | 4 (11%) | Ns‡ | |
| Grade 3/4 | 1 (10%) | 2 (5%) | ||
| TP (g dL-1) | 7.18 ± 0.63 | 7.15 ± 0.59 | Ns† | |
| Alb (g dL-1) | 3.88 ± 0.41 | 4.00 ± 0.44 | Ns† | |
| WBC (uL-1) | 9400 ± 4500 | 8000 ± 3500 | Ns† | |
| Hb (g dL-1) | 13.2 ± 1.34 | 13.0 ± 1.57 | Ns† | |
| Plt (×103 uL-1) | 304 ± 110 | 260 ± 63 | Ns† | |
| Cr (mg dL-1) | 0.63 ± 0.10 | 0.73 ± 0.19 | Ns† | |
| LDH (U L-1) | 257 ± 137 | 226 ± 71.4 | Ns† | |
| CRP (mg dL-1) | 1.73 ± 1.76 | 1.15 ± 1.44 | Ns† | |
*Adverse event was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Event (version 4.0).
†Mann-Whitney test; ‡chi-square test.
Alb, albumin; CINV, chemotherapy-induced nausea and vomiting; Cr, creatinine; CRP, c-reactive protein; Hb, hemoglobin; LDH, lactic acid dehydrogenase; n, number of cases (% total cases); Ns, not significant; PD, progressive disease; Plt, platelet; PR, partial response; SD, stable disease; TP, total protein; WBC, white blood cell.
Table 4.
Body weight change in the study patients and retrospective controls
| Patients | Retrospective controls | Unadjusted analysis* | IPTW analysis† | ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||||
| n | 10 | 38 | |||||||
| Body weight change | Gain | 6 (60%) | 8 (21%) | 5.6 | 1.2–25 | 0.044 | 8.4 | 1.6–42 | 0.01 |
| Loss | 4 (40%) | 30 (79%) | |||||||
IPTW analysis for the study patients versus retrospective controls was estimated using a logistic regression model based on age, sex, chemotherapy (platinum-based doublet chemotherapy or non-platinum chemotherapy), body height, and body weight.
*chi-square test; †generalized estimating equations.
CI, confidence interval; IPTW, inverse probability of treatment weight; OR, odds ratio.
DISCUSSION
Chemotherapy-induced weight loss is among the serious problems in cancer treatment. The present study demonstrated that individualized intensive nutrition intervention reduced weight loss during the course of cytotoxic chemotherapy. Furthermore, the body weight change of the study cohort was significantly different with that of the retrospective controls on IPTW analysis. The patients received adequate antiemetic drugs and high-quality nutritional information and education, thereby reducing weight loss during cytotoxic chemotherapy.
In previous studies, nutrition interventions such as DC and/or ONS yielded no improvement in body weight, 9–11 but ONS with EPA and DHA-enriched supplementation resulted in a significant improvement in patients with cancer during chemotherapy.8, 31–33 Meanwhile, in the current study, nutrition intervention without EPA and DHA-enriched supplement caused increased body weight in the patients compared with the retrospective control. This outcome might be due to two reasons. First, the control of CINV has considerably improved in the past 10 years. The availability of aprepitant in addition to other antiemetic agents, such as 5-HT3RA and dexamethasone, has improved treatment outcomes of CINV. Currently, 3-drug regimens (aprepitant, 5-HT3RA and dexamethasone) are strongly recommended to prevent CINV during HEC.16–19 Consequently, the present study shows that appropriate nutrition intervention with antiemetic drugs prevented reduced dietary intake and body weight from CINV. Second, dietary intake of EPA and DHA among Japanese is generally higher than that in other populations. EPA and DHA-enriched supplementation8, 31–33 causes weight gain as they can downregulate the production of pro-inflammatory cytokines and mediate the modulation of systemic inflammation.34, 8, 31–35 As such, EPA and DHA reduce anorexia, resting energy expenditure, muscle degradation and weight loss.8, 31–36–39 The EPA and DHA intake of Japanese is approximately four-fold higher compared to that of Americans. Based on the results of the 2005 and 2006 NHNS,40 the median EPA and DHA intake of Japanese aged 30–49 years is 0.32 g/day in men and 0.23 g/day in women, while it is 0.086 g/day in men and 0.063 g/day in women among Americans aged 31–50 years.41 In this study, the consumption of EPA at baseline and after 90-day intervention were 0.22 ± 0.13 g/day and 0.42 ± 0.26 g/day and DHA at baseline and after 90-day intervention were 0.38 ± 0.18 g/day and 0.56 ± 0.38 g/day, respectively. The patients took adequate amounts of EPA and DHA daily. For patients who take enough fish oil like the Japanese, nutrition intervention may have strong effect on preventing weight loss even without EPA and DHA supplementation.
Weight loss in cancer patients administered chemotherapy consists of chemotherapy-induced weight loss and cancer cachexia. These two are interconnected and inseparable. The efficacy of nutritional intervention on cancer cachexia was not assessed in this study. In a short period of 90-day intervention, the patients had increased BMI, while they did not gain SMI, and had reduced grip strength. Patients with muscle depletion and low muscle attenuation have worse prognoses independent of other factors.42 The importance of rehabilitation and exercise for cancer patients has been recently recognized. Vigorous exercise and physical activity were associated with lower risk of colorectal cancer-related overall mortality,43 prostate cancer progression,44 and reduced symptoms of lung cancer.45 Future research should pay attention to not only nutrition intervention but also physical intervention, such as rehabilitation or exercise.
In conclusion, early intensive nutritional intervention during chemotherapy resulted in maintenance of body weight compared with patients who received standard care. With the development of antiemetic drugs to prevent CINV, nutrition interventions such as DC and ONS might be beneficial not only for body weight but also clinically relevant outcomes such as QOL, treatment response and survival.
LIMITATIONS
This study has some limitations. First, although improvements in clinically relevant outcomes such as treatment response and survival are primary targets for nutrition intervention, the effects of these outcomes had not been assessed in this study. Second, the number of patients was too small, and this study was single arm. Larger randomized trials are required in the future.
Acknowledgments
Acknowledgments: This study was supported by grants from Tottori University, Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Shionogi & Co., Ltd., and Nippon Kayaku Co., Ltd. We offer our sincere thanks to Naoko Endo and Natsuko Nakayama (Division of Nutrition Management, Tottori University Hospital, Yonago, Japan). We are also deeply grateful to Dr. Hisashi Noma (Department of Data Science, The Institute of Statistical Mathematics, Tokyo, Japan) for his support in statistical analysis.
The authors declare no conflict of interest.
REFERENCES
- 1. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491-7. [DOI] [PubMed] [Google Scholar]
- 2. Laviano A, Meguid MM. Nutritional issues in cancer management. Nutrition. 1996;12:358-71. [DOI] [PubMed] [Google Scholar]
- 3. Bennani-Baiti N, Davis MP. Cytokines and cancer anorexia cachexia syndrome. Am J Hosp Palliat Care. 2008;25:407-11. [DOI] [PubMed] [Google Scholar]
- 4. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clinical nutrition (Edinburgh, Scotland). 2017;36:11-48. [DOI] [PubMed] [Google Scholar]
- 5. Lorusso V, Giampaglia M, Petrucelli L, Saracino V, Perrone T, Gnoni A. Antiemetic efficacy of single-dose palonosetron and dexamethasone in patients receiving multiple cycles of multiple day-based chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:3241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:843-51. [DOI] [PubMed] [Google Scholar]
- 7. Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472-8. [DOI] [PubMed] [Google Scholar]
- 8. de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29:1141-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, Germain C, Blanc JF, Dauba J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9:e108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24:431-40. [DOI] [PubMed] [Google Scholar]
- 11. Elkort RJ, Baker FL, Vitale JJ, Cordano A. Long-term nutritional support as an adjunct to chemotherapy for breast cancer. JPEN J Parenter Enteral Nutr. 1981;5:385-90. [DOI] [PubMed] [Google Scholar]
- 12. Chawla SP, Grunberg SM, Gralla RJ, Hesketh PJ, Rittenberg C, Elmer ME, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:2290-300. [DOI] [PubMed] [Google Scholar]
- 13. Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol.. 2003;21:4112-9. [DOI] [PubMed] [Google Scholar]
- 14. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090-8. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K, et al. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci. 2010;101:2455-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeuchi H, Saeki T, Aiba K, Tamura K, Aogi K, Eguchi K, et al. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016;21:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw. 2017;15:883-93. [DOI] [PubMed] [Google Scholar]
- 18. Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27:v119-v33. [DOI] [PubMed] [Google Scholar]
- 19. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3240-61. [DOI] [PubMed] [Google Scholar]
- 20. Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4:370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr. 1979;3:452-6. [DOI] [PubMed] [Google Scholar]
- 22. Takayanagi T, Sasaki H, Kawashima A, Mizuochi Y, Hirate H, Sugiura T, et al. A new enteral diet, MHN-02, which contains abundant antioxidants and whey peptide, protects against carbon tetrachloride-induced hepatitis. JPEN J Parenter Enteral Nutr. 2011;35:516-22. [DOI] [PubMed] [Google Scholar]
- 23. Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53-72. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14:1200-11. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22:151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310:2191-4. [DOI] [PubMed] [Google Scholar]
- 28. Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11:80-6. [DOI] [PubMed] [Google Scholar]
- 29. Simons JP, Schols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin sci (Lond). 1999;97:215-23. [PubMed] [Google Scholar]
- 30. Finocchiaro C, Segre O, Fadda M, Monge T, Scigliano M, Schena M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. The British journal of nutrition. 2012;108:327-33. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Lara K, Turcott JG, Juarez-Hernandez E, Nunez-Valencia C, Villanueva G, Guevara P, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clinical nutrition (Edinburgh, Scotland). 2014;33:1017-23. [DOI] [PubMed] [Google Scholar]
- 32. Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol. 2003;21:129-34. [DOI] [PubMed] [Google Scholar]
- 33. van der Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, von Blomberg BM, Heijboer AC, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 2010;140:1774-80. [DOI] [PubMed] [Google Scholar]
- 34. de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clinical nutrition (Edinburgh, Scotland). 2015;34:359-66. [DOI] [PubMed] [Google Scholar]
- 35. Laviano A, Rianda S, Molfino A, Rossi Fanelli F. Omega-3 fatty acids in cancer. Curr Opin Clin Nutr Metab Care. 2013;16:156-61. [DOI] [PubMed] [Google Scholar]
- 36. Wigmore SJ, Fearon KC, Maingay JP, Ross JA. Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin sci (Lond). 1997;92:215-21. [DOI] [PubMed] [Google Scholar]
- 37. Barber MD, McMillan DC, Preston T, Ross JA, Fearon KC. Metabolic response to feeding in weight-losing pancreatic cancer patients and its modulation by a fish-oil-enriched nutritional supplement. Clin sci (Lond). 2000;98:389-99. [PubMed] [Google Scholar]
- 38. Barber MD, Ross JA, Preston T, Shenkin A, Fearon KC. Fish oil-enriched nutritional supplement attenuates progression of the acute-phase response in weight-losing patients with advanced pancreatic cancer. J Nutr. 1999;129:1120-5. [DOI] [PubMed] [Google Scholar]
- 39. Wigmore SJ, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000;36:177-84. [DOI] [PubMed] [Google Scholar]
- 40. Fukuda Y. The National Health and Nutrition Survey in Japan, 2016 [Internet]. Ministry of Health, Labor and Welfare; [cited 2018 Aug 15]. Available from: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku-04.pdf. Japanese. [Google Scholar]
- 41. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621-30. [DOI] [PubMed] [Google Scholar]
- 42. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-47. [DOI] [PubMed] [Google Scholar]
- 43. Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bade BC, Thomas DD, Scott JB, Silvestri GA. Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol. 2015;10:861-71. [DOI] [PubMed] [Google Scholar]