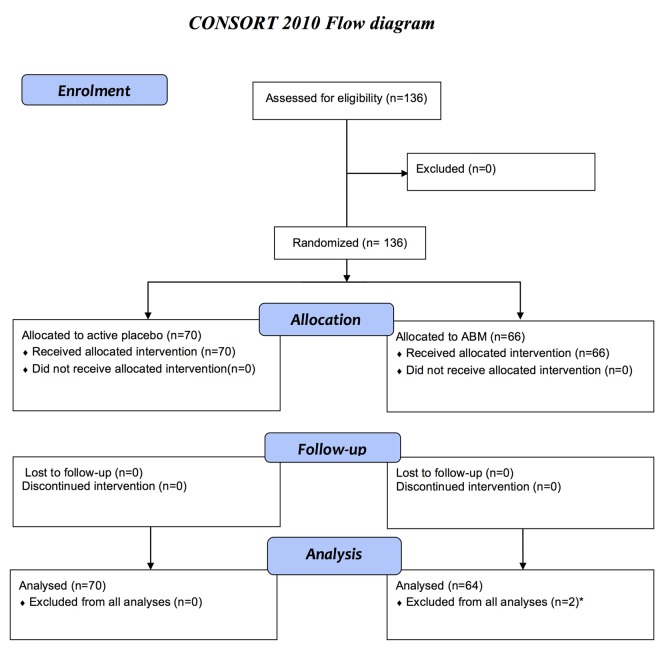
Figure 1.
Flow diagram for enrolment, allocation to active placebo or attentional bias modification (ABM), follow-up after 2 weeks, and analyses in accordance with consort. The current clinical trial (NCT02931487) is an extension of a larger double-blinded randomized clinical trial (RCT; NCT02658682) including 321 patients with a history of depression. The current study is based on a subsample from the main RCT. Only participants that fulfilled the inclusion criteria per protocol was invited to participate in the magnetic resonance imaging (MRI) study, from May 2015 to December, 2016. The sample consists of 136 participants that agreed to participate in the MRI study and had no contraindication for MRI scanning. A total of 134 eligible participants between 18 years and 65 years old were included in MRI analyses. *Excluded due to technical problems with the head coil.