Abstract
Introduction
Shrunken pore syndrome (SPS), originally defined by cystatin C−based estimated glomerular filtration rate (eGFRcystatin C) being less than 60% of creatinine-based estimated glomerular filtration rate (eGFRcreatinine) in the absence of extrarenal influences on the plasma levels of cystatin C or creatinine, is associated with a high increase in mortality, even in the absence of reduced glomerular filtration rate (GFR). The objective of the present study was to determine whether the proteome of patients with SPS shows differences from that of patients with normal or reduced measured GFR (mGFR) without SPS.
Methods
Four patient cohorts were included: 1 cohort with normal mGFR without SPS, 1 with normal mGFR with SPS, 1 with reduced mGFR without SPS, and 1 with reduced mGFR with SPS. The plasma levels of 177 selected proteins were analyzed.
Results
Differences in the levels of 30 proteins were specific for SPS; 31 differences were specific for patients with both SPS and reduced mGFR; and 27 were specific for reduced mGFR. Eighteen of the differences specific for SPS concerned proteins described as promoting, or being associated with, atherosclerosis. Twelve of the differences specific for patients with both SPS and reduced mGFR and 10 of the differences specific for reduced mGFR also concerned proteins described as promoting, or being associated with, atherosclerosis. Almost all (82 of 88) of the concentration differences represented increased levels. For SPS, but not for reduced mGFR, a correlation between protein size and increase in level was observed, with smaller proteins being associated with higher levels.
Conclusion
The high mortality in shrunken pore syndrome might be caused by the accumulation of atherosclerosis-promoting proteins in this condition.
Keywords: atherosclerosis, creatinine, cystatin C, GFR, kidney, mortality
Chronic kidney disease (CKD) is a public health problem affecting millions of people worldwide and is an independent risk factor for cardiovascular disease and premature death.1 The diagnosis of CKD generally involves the use of a cystatin C−based or a creatinine-based glomerular filtration rate (GFR) estimating equation, since measured GFR (mGFR) can be obtained only by use of an invasive procedure. The mean of a cystatin C−based and a creatinine-based GFR estimating equation usually produces the most reliable GFR estimate.2, 3, 4 Although the cystatin C−based or creatinine-based GFR estimates generally agree, they disagree in some cases in the absence of known extrarenal influences on the plasma levels of cystatin C or creatinine. In virtually all of these cases, a cystatin C−based estimated GFR (eGFRcystatin C) is lower than a creatinine-based eGFR (eGFRcreatinine). It has been suggested that an eGFRcystatin C/eGFRcreatinine ratio ≤0.60 represents a new syndrome, called shrunken pore syndrome (SPS),5 as the glomerular filtration of 12- to 29-kDa molecules seemed to be selectively impaired.4, 5 The long-term mortality6, 7, 8 and morbidity8, 9 of patients with SPS have been shown to be strongly increased in several cohorts, even in the absence of reduced GFR. Moreover, cardiovascular manifestations have represented a major part of the mortality.6, 7, 8, 9, 10 All of these studies have used cystatin C−based and/or creatinine-based GFR estimating equations to estimate GFR, but in an ongoing investigation of 2805 patients with measured GFR, SPS is also associated with a markedly shortened survival (A. Grubb, J. Björk, unpublished data). In the present work, the plasma levels of 177 selected proteins were determined in 156 patients from this cohort, with or without SPS and with or without reduced mGFR, to examine whether the proteome of patients with SPS differs from that of patients with normal or reduced mGFR in the absence of SPS. A second objective was to investigate whether some of the alterations in protein levels might contribute to an understanding of the increased mortality of patients with SPS. A third objective was to investigate the relationship between protein size and plasma level in patients with SPS and in patients with reduced mGFR in the absence of SPS.
Materials and Methods
Study Population
The cohort of 156 patients studied in this work was selected from the Lund Cystatin C Standardization (LCS) cohort, primarily established to generate a new cystatin C-based GFR estimation equation (the CAPA equation).11 The LCS cohort is based on consecutive Swedish Caucasian (≥99%) patients above 18 years referred for determination of GFR by iohexol clearance at Skåne University Hospital, Lund, Sweden, from May 2008 to March 2010. During this period, 3495 GFR determinations were performed in 2847 patients. Common causes for referral were manifest or suspected diabetic nephropathy, interstitial nephritis, glomerulonephritis, nephrotic syndrome, hematuria, proteinuria, reflux nephropathy, myeloma, vasculitis, consideration of initiation of hemodialysis, evaluation of potential renal donors, control after kidney transplantation, and dosing of drugs cleared by the kidneys. For the present study, only data from the patients’ first mGFR examination were included. A total of 2805 unique patients could be identified and followed longitudinally from the date of their first examination. The patients were, among other things, characterized concerning diagnosis at referral to the laboratory, mortality, death cause, and plasma level of cystatin C and creatinine.11 From this cohort, 4 subcohorts of 39 individuals were selected, representing patients with normal mGFR, with or without SPS, and patients with reduced mGFR, with or without SPS. The patients were selected so that those with and without SPS did not differ significantly in terms of mGFR, age, body mass index (BMI), weight, or percentage of males/females (Table 1), whether the patients had normal mGFR (≥60 ml/min per 1.73 m2) or reduced mGFR (<60 ml/min per 1.73 m2). For each patient, the plasma levels of 177 selected proteins were measured. The study was approved by the local ethics committee (permissions LU 2015/860 and 2016/169). Patient data and samples were treated anonymously in all statistical analyses.
Table 1.
Basic characteristics of the 4 cohorts
| Cohort characteristics | SPS mGFR ≥60 ml/min per 1.73 m2 | No SPS mGFR ≥60 ml/min per 1.73 m2 | SPS mGFR <60 ml/min per 1.73 m2 | No SPS mGFR <60 ml/min per 1.73 m2 |
|---|---|---|---|---|
| Number of patients | 39 | 39 | 39 | 39 |
| Age, yr | 58 (29, 75) | 57 (23, 82) | 67 (33, 83) | 65 (27, 85) |
| Weight, kg | 73 (46, 127) | 74 (52, 102) | 75 (44, 107) | 79 (53, 99) |
| Body mass index | 24 (17, 37) | 25 (19, 33) | 25 (17, 37) | 27 (20, 32) |
| Females, % | 48.7 | 48.7 | 38.5 | 35.9 |
| mGFR, ml/min per 1.73 m2 | 74 (60, 110) | 83 (62, 112) | 30 (6, 57) | 35 (12, 56) |
| Cystatin C, mg/l | 1.68a (1.21, 2.27) | 0.95 (0.77, 1.29) | 3.02a (1.80, 6.75) | 2.16 (1.28, 3.96) |
| Creatinine, μmol/lb | 59a (37, 110) | 78 (52, 110) | 121a (65, 495) | 162 (94, 410) |
| CAPAcystatin C ml/min per 1.73 m2 | 40a (26, 60) | 80 (55, 109) | 17a (3, 37) | 28 (13, 61) |
| LM-REVcreatininec ml/min per 1.73 m2 | 92a (56, 122) | 78 (55, 104) | 38a (10, 82) | 28 (13, 60) |
| CAPAcystatin C /c LM-REVcreatinine ratio |
0.47a (0.33, 0.55) | 1.00 (0.95, 1.05) | 0.40a (0.31, 0.55) | 1.00 (0.95, 1.05) |
| All-cause mortality during 5 years, % | 56.4a | 28.2 | 74.4a | 28.2 |
Continuous characteristics are presented as median (2.5−97.5 percentiles); categorical values are presented as percentage (%). Differences between groups were tested using a nonparametric method (Mann-Whitney U test). A p value <0.05 was considered significant. CAPAcystatin C, eGFR using the Caucasian-Asian-Pediatric-Adult equation11; LM-REVcreatinine, eGFR using Lund-Malmö-revised equation18; mGFR, measured glomerular filtration rate.
Statistical differences between parameters of patients with and without SPS at mGFR ≥ or <60 ml/min per 1.73 m2.
Conversion factor: μmol/l divided by 88.4 = mg/dl.
Both CAPAcystatin C and LM-REVcreatinine can be determined by using the tool available at www.egfr.se.
Measurement of GFR
Glomerular filtration rate was measured as plasma clearance of iohexol.12 A recent systematic review of methods to measure GFR has shown that this method produces results comparable to those based on measuring urinary clearance of inulin.13
Measurements of Protein and Creatinine Concentrations
Relative protein levels of 177 proteins were measured using Olink CARDIOVASCULAR II and Olink INFLAMMATION panels (Olink Proteomics AB, Uppsala, Sweden) according to the manufacturer’s instructions. The Proximity Extension Assay (PEA) technology used for the Olink protocol has been well described14 and enables 92 analytes to be analyzed simultaneously, using 1 μl of each sample. In brief, pairs of oligonucleotide-labeled antibody probes bind to their targeted protein, and if the 2 probes are brought in close proximity, the oligonucleotides will hybridize in a pairwise manner. The addition of a DNA polymerase leads to a proximity-dependent DNA polymerization event, generating a unique polymerase chain reaction target sequence. The resulting DNA sequence is subsequently detected and quantified using a microfluidic real-time polymerase chain reaction instrument (Biomark HD, Fluidigm, South San Francisco, CA). Data are then quality controlled and normalized using an internal extension control and an interplate control, to adjust for intra- and interrun variation. Quality control for each sample was performed following Olink’s recommended thresholds for the included control assays of the antibody incubation and quantitative polymerase chain reaction detection. Based on this assessment, 5 samples were excluded from the CARDIOVASCULAR II panel and 2 samples from the INFLAMMATION panel in all statistical analyses, leaving 151 and 154 samples, respectively (see Supplementary Table S1 for the number of samples used in the analysis for each assay). The Olink Proteomics platform measures protein levels in a unit called Normalized Protein eXpression (NPX) that is a relative measurement on log2 scale (1 NPX change translates approximately into a 2-fold change in protein concentration). The NPX scale is recommended by Olink Proteomics for all statistical analysis and was used throughout the study. More information about NPX and the Olink platform, including all assay validation data (detection limits, intra- and interassay precision data, etc.), are available on the manufacturer’s website (www.olink.com).
The plasma cystatin C levels in all samples were determined by an automated particle-enhanced immunoturbidimetric method15 using a reference material traceable to the international cystatin C calibrator.16 Creatinine levels were determined by an enzymatic colorimetric assay using a calibrator traceable to primary reference material with values assigned by isotope dilution mass spectrometry.17
Statistical Analysis
Differences in the characteristics of the 4 studied cohorts (Table 1) were tested using a nonparametric method (Mann−Whitney U test). A P value <0.05 was considered significant.
We investigated the impact of SPS, reduced mGFR, and their potential interaction for each protein using age, sex, and BMI by fitting a full linear model (NPX ∼ SPS + mGFR + SPS * mGFR + Age + Sex +BMI). Statistical significance for each term was determined by the Wald test, and the P values were corrected for multiple testing using Benjamini−Hochberg’s method. An adjusted P value <0.05 was considered statistically significant. Significant changes in protein levels were assigned into 4 classes depending on the detected changes with SPS and mGFR: (i) SPS specific, (ii) reduced mGFR specific, (iii) specific for simultaneous presence of SPS and reduced mGFR, and (iv) interaction of SPS and mGFR. Estimated coefficients for the main effect of SPS and mGFR have essentially the same values (median linear difference, 5%) when comparing the full linear model with a model excluding the interaction term. To assess whether protein size (Dalton) correlates with the observed changes in plasma concentration in patients with SPS or with reduced mGFR, linear regression was used. A model was fitted for estimated effects (ΔNPX ∼ Size) for both mGFR and SPS that included all significant changes in protein levels (P < 0.05, Wald test) from the analysis above. For easier interpretation, the estimated coefficients were linearized to percentage difference in all figures and Table 2 by calculating 100 × (2c – 1), where c is the coefficient. The differences are relative to patients with normal mGFR (≥60 ml/min per 1.73 m2) and without SPS. Please note that the linearized relative values are multiplicative and not additive. All statistical analyses of Olink proteomics data were carried out using the R statistical programming language (R Foundation for Statistical Computing, Vienna, Austria).
Table 2.
Proteins for which there were changes in plasma protein levels specific for shrunken pore syndrome (SPS), specific for reduced measured GFR (rGFR), or occurring both in SPS and in rGFR compared to patients with normal measured GFR without SPS
| Protein | Condition | References for atherosclerosis association | Full protein name |
|---|---|---|---|
| MCP-3 | SPS | 38 | Monocyte chemotactic protein–3 |
| CDCP1 | SPS | CUB domain-containing protein 1 | |
| ADAM-TS13 | SPS | 39 | A disintegrin and metalloproteinase with thrombospondin motifs 13 |
| IL-4RA | SPS | Interleukin-4 receptor subunit α | |
| OPG | SPS | 40 | Osteoprotegerin |
| IL-1ra | SPS | 41 | Interleukin-1 receptor antagonist protein |
| IL-6 | SPS | 42, 43 | Interleukin-6 |
| IL-17C | SPS | 44 | Interleukin-17C |
| MCP-1 | SPS | 45 | Monocyte chemoattractant protein–1 |
| CXCL11 | SPS | 46 | C-X-C motif chemokine 11 |
| IL-18 | SPS | 47 | Interleukin-18 |
| FGF-21 | SPS | Fibroblast growth factor 21 | |
| TGFA | SPS | Protransforming growth factor α | |
| CCL19 | SPS | 48 | C-C motif chemokine 19 |
| IL-18R1 | SPS | 49 | Interleukin-18 receptor 1 |
| PD-L1 | SPS | 50 | Programmed cell death 1 ligand 1 |
| HGF | SPS | 51, 52 | Hepatocyte growth factor |
| HO-1 | SPS | Heme oxygenase 1 | |
| IL-10 | SPS | Interleukin-10 | |
| PTX3 | SPS | 53 | Pentraxin 3 |
| CXCL10 | SPS | 46, 54, 55 | C-X-C motif chemokine 10 |
| 4E-BP1 | SPS | 56 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| GDF-2 | SPS | Growth/differentiation factor 2 | |
| MCP-2 | SPS | C-C motif chemokine 8 | |
| CTSL1 | SPS | 57 | Cathepsin L1 |
| CA5A | SPS | Carbonic anhydrase 5A, mitochondrial | |
| CCL20 | SPS | 58, 59 | C-C motif chemokine 20 |
| ADA | SPS | Adenosine deaminase | |
| PARP-1 | SPS | Poly [ADP-ribose] polymerase 1 | |
| HAOX1 | SPS | Hydroxyacid oxidase 1 | |
| VEGF-A | SPS and rGFR | Vascular endothelial growth factor A | |
| ADM | SPS and rGFR | 60 | Adrenomedullin |
| PlGF | SPS and rGFR | 61 | Placenta growth factor |
| TNFRSF10A | SPS and rGFR | Tumor necrosis factor receptor superfamily member 10A | |
| TNFRSF11A | SPS and rGFR | Tumor necrosis factor receptor superfamily member 11A | |
| TRAIL-R2 | SPS and rGFR | 62 | Tumor necrosis factor-related apoptosis-inducing ligand receptor 2 |
| CXCL9 | SPS and rGFR | C-X-C motif chemokine 9 | |
| IL27 | SPS and rGFR | 63 | Interleukin 27 |
| SCF | SPS and rGFR | Kit ligand | |
| SLAMF1 | SPS and rGFR | Signaling lymphocytic activation molecule | |
| LIF-R | SPS and rGFR | Leukemia inhibitory factor receptor | |
| IL-15RA | SPS and rGFR | Interleukin-15 receptor subunit α | |
| IL-10RB | SPS and rGFR | Interleukin-10 receptor subunit β | |
| REN | SPS and rGFR | 64 | Renin |
| MERTK | SPS and rGFR | Tyrosine-protein kinase Mer | |
| TIM | SPS and rGFR | Hepatitis A virus cellular receptor 1 | |
| TM | SPS and rGFR | 65 | Thrombomodulin |
| VSIG2 | SPS and rGFR | V-set and Ig domain-containing protein 2 | |
| IL16 | SPS and rGFR | Pro-interleukin-16 | |
| MMP-10 | SPS and rGFR | 66 | Matrix metalloproteinase 10 |
| CCL23 | SPS and rGFR | 67 | C-C motif chemokine 23 |
| PRSS8 | SPS and rGFR | Prostasin | |
| AGRP | SPS and rGFR | Agouti-related protein | |
| CD40 | SPS and rGFR | 68 | Tumor necrosis factor receptor superfamily member 5 |
| PD-L2 | SPS and rGFR | Programmed cell death 1 ligand 2 | |
| CX3CL1 | SPS and rGFR | 69 | Fractalkine |
| hOSCAR | SPS and rGFR | 70 | Osteoclast-associated Ig-like receptor |
| TNFRSF9 | SPS and rGFR | 71 | Tumor necrosis factor receptor superfamily member 9 |
| CSF-1 | SPS and rGFR | Macrophage colony-stimulating factor 1 | |
| DCN | SPS and rGFR | Decorin | |
| SLAMF7 | SPS and rGFR | SLAM family member 7 | |
| SRC | rGFR | Proto-oncogene tyrosine-protein kinase Src | |
| PRSS27 | rGFR | Serine protease 27 | |
| CST5 | rGFR | Cystatin-D | |
| TF | rGFR | 72 | Tissue factor |
| IL-17D | rGFR | Interleukin-17D | |
| RAGE | rGFR | 73 | Advanced glycosylation end product-specific receptor |
| TNFSF14 | rGFR | Tumor necrosis factor ligand superfamily member 14 | |
| FGF-23 | rGFR | 74, 75 | Fibroblast growth factor 23 |
| SPON2 | rGFR | Spondin-2 | |
| FGF-5 | rGFR | Fibroblast growth factor 5 | |
| β-NGF | rGFR | β-Nerve growth factor | |
| AMBP | rGFR | α-1-Microglobulin/bikunin precursor | |
| IL-12B | rGFR | Interleukin-12 subunit β | |
| PRELP | rGFR | Prolargin | |
| XCL1 | rGFR | Lymphotactin | |
| CD5 | rGFR | T-cell surface glycoprotein CD5 | |
| MMP-7 | rGFR | 76 - 78 | Matrix metalloproteinase-7 |
| LPL | rGFR | 79 | Lipoprotein lipase |
| HB-EGF | rGFR | 80 | Proheparin-binding EGF-like growth factor |
| FABP2 | rGFR | 81 | Fatty acid-binding protein 2 |
| GT | rGFR | Gastrotropin | |
| CASP-8 | rGFR | Caspase-8 | |
| CCL25 | rGFR | 82 | chemokine receptor 9-chemokine ligand 25 |
| TNFRSF13B | rGFR | Tumor necrosis factor receptor superfamily member 13B | |
| LEP | rGFR | 83 | Leptin |
| CD4 | rGFR | T-cell surface glycoprotein CD4 | |
| VEGF-D | rGFR | 84 | Vascular endothelial growth factor D |
Results
A large population of Swedish, Dutch, and Japanese adults and children with measured GFR was recently used to generate a cystatin C−based GFR estimating equation useful for all ages (the Caucasian, Asian, Pediatric and Adult, or CAPA, equation).11 Of the Swedish adults, 2805 have been followed for at least 5 years. For the present study, 156 patients were selected, comprising 4 subcohorts of 39 patients, defined as follows: (i) normal mGFR (≥60 ml/min per 1.73 m2) with SPS; (ii) normal mGFR without SPS; (iii) reduced mGFR (<60 ml/min per 1.73 m2) with SPS; and (iv) reduced mGFR without SPS. The cohorts were selected so that age, gender composition, BMI or weight, and mGFR were not significantly different between the groups with or without SPS for normal or reduced mGFR. The basic characteristics of these cohorts are given in Table 1, including the estimated GFR obtained using the estimating equations CAPAcystatin C11 and LM-REVcreatinine18 and the eGFRcystatin C/eGFRcreatinine ratios using these equations. An eGFRcystatin C/eGFRcreatinine ratio of ≤0.60 was used as a cut-off to define the presence or absence of SPS.5 As expected, there were significant differences in the levels of cystatin C or creatinine, CAPAcystatin C, LM-REVcreatinine, and the eGFRcystatin C/eGFRcreatinine ratio between patients with and without SPS for both normal and reduced mGFR. The survival after 5 years was ≤50% in the populations with SPS compared to the populations without SPS, independent of whether mGFR was normal (P = 0.0123) or reduced (P < 0.0001) (Table 1). The major causes of death were cardiovascular disorders or cancer and the proportion of cardiovascular causes of death were greater in the groups with SPS; however, the differences were not statistically significant, probably due to the low number of patients studied.
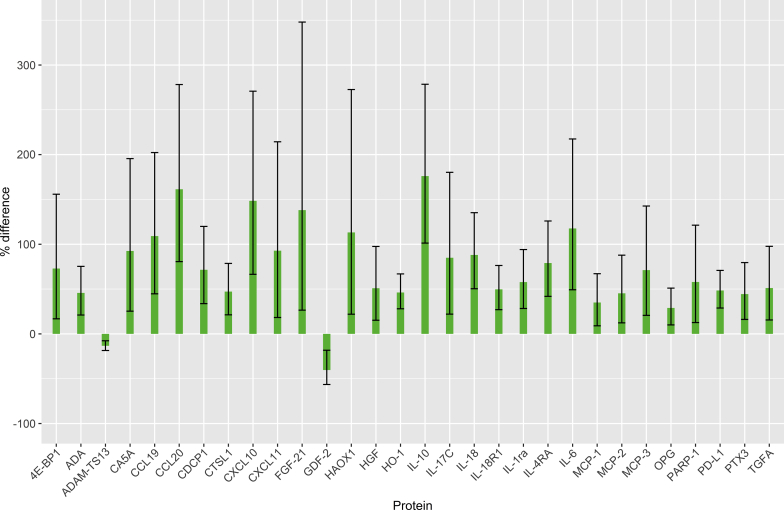
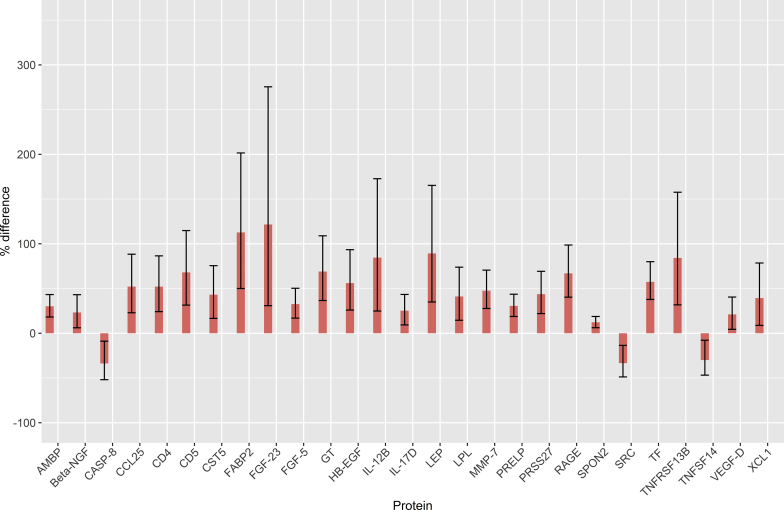
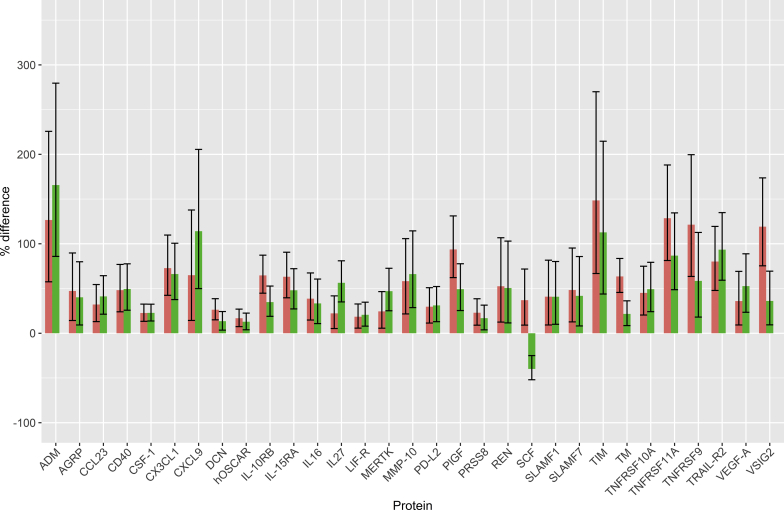
By measuring the relative plasma levels of 177 proteins in the 4 cohorts using Olink's proteomics platform (Supplementary Table S1) based on the proximity extension assay,14 we could detect significant differences in the levels of 88 proteins (multiple test adjusted P < 0.05) associated with SPS and/or reduced mGFR. Of these 88 differences, 30 were specifically associated with SPS, 27 with reduced mGFR, and 31 with both SPS and reduced mGFR in a regression model. Of the 30 differences specifically associated with SPS, 28 concerned increased levels (Figure 1). Of the 27 differences specifically associated with reduced mGFR, 24 represented increases (Figure 2). Of the 31 differences associated with both SPS and reduced mGFR, 30 represented increases (Figure 3). Three proteins had a significant interaction term indicating that they had a specific change with the simultaneous presence of SPS and reduced mGFR (Supplementary Table S1).
Figure 1.
Protein concentration changes (%) in patients with shrunken pore syndrome (SPS). Changes are relative to the concentrations in patients without SPS and with normal measured glomerular filtration rate (mGFR). Error bars represent 95% confidence intervals for the estimated changes. Protein concentration changes were estimated from the coefficients of a full linear model (n = 154/151; see Supplementary Table S1 for each assay). Full protein names are given in Table 2.
Figure 2.
Protein concentration changes (%) in patients with reduced mGFR. Changes are relative to the concentrations in patients without shrunken pore syndrome (SPS) and with normal measured glomerular filtration rate (mGFR). Error bars represent 95% confidence intervals for the estimated changes. Protein concentration changes were estimated from the coefficients of a full linear model (n = 154/151; see Supplementary Table S1 for each assay). Full protein names are given in Table 2.
Figure 3.
Protein concentration changes (%) in patients with both shrunken pore syndrome (SPS) (green) and reduced mGFR (red). Changes are relative to the concentrations in patients without SPS and with normal mGFR. Error bars represent 95% confidence intervals for the estimated changes. Protein concentration changes were estimated from the coefficients of a full linear model (n = 154/151; see Supplementary Table S1 for each assay). Full protein names are given in Table 2.
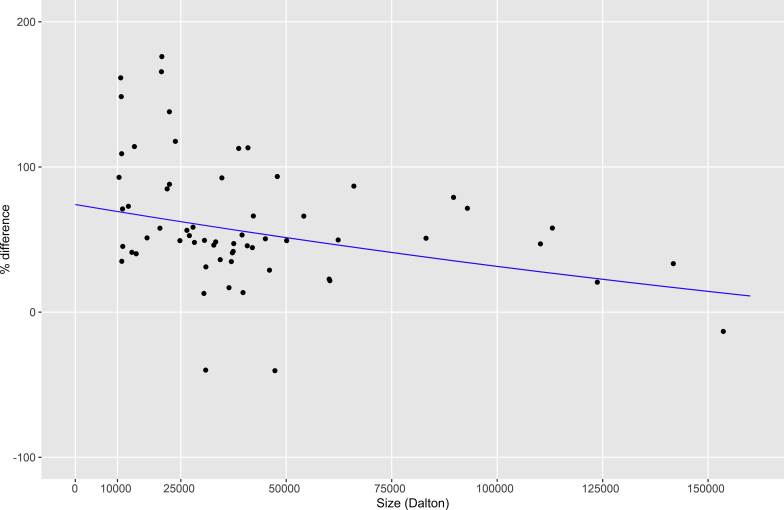
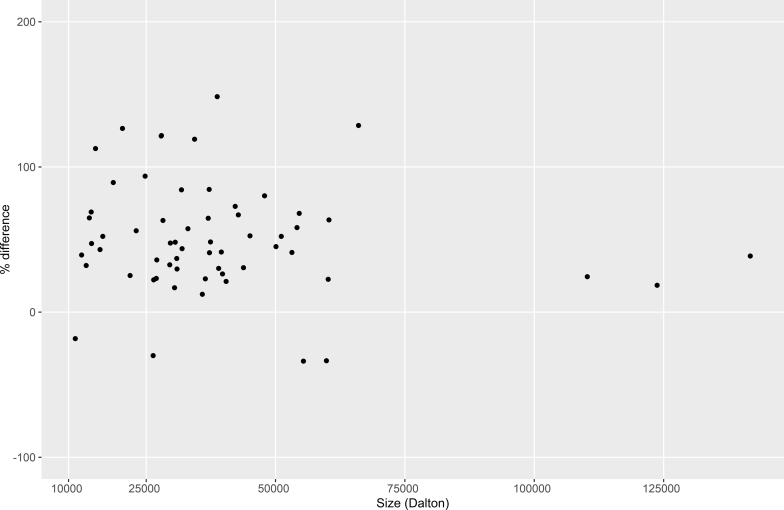
It has previously been noted that there seems to be a tendency toward increased levels of smaller rather than larger proteins in SPS.5, 7, 19 We therefore tested whether the size of the proteins in this study (approximated as molecular mass in Daltons) influenced their changes in concentration, using linear regression. For SPS, a significant inverse correlation (P < 0.05) was found between concentration change and protein size, with higher increases in the levels of smaller proteins (Figure 4). No such correlation (P = 0.50) could be seen for reduced mGFR (Figure 5).
Figure 4.
Correlation (P < 0.05) between protein concentration change in patients with shrunken pore syndrome and protein size approximated as molecular mass in Daltons. Protein concentration changes were estimated from the coefficients of a full linear model (n = 154/151; see Supplementary Table S1 for each assay). Only proteins with significant concentration changes were included in the analysis.
Figure 5.
No correlation (P = 0.29) could be seen between protein concentration change in patients with reduced glomerular filtration rate (GFR) and protein size approximated as molecular mass in Daltons. Protein concentration changes were estimated from the coefficients of a full linear model (n = 154/151; see Supplementary Table S1 for each assay). Only proteins with significant concentration changes were included in the analysis.
Discussion
Reduced GFR has been known to increase the plasma level of cystatin C since 197920 and cystatin C has been used as a marker for GFR since 1985.21 The introduction of an automated procedure for measurement of cystatin C in 199415 has led to widespread use of cystatin C, or cystatin C−based estimating equations, to assess GFR. Cystatin C is commonly superior to creatinine as a marker of GFR22; however, if creatinine-based GFR estimating equations also incorporate age, sex, and race factors, they are generally comparable to cystatin C−based equations using only cystatin C as a parameter.4, 23 A recent study of the levels of 2893 proteins in plasma demonstrated that the cystatin C level was the one most strongly correlated with measured GFR.24 A decrease in mGFR or eGFR signals increased risks for development of end-stage renal disease, cardiovascular manifestations, hospitalization, and death. An eGFR based on cystatin C (eGFRcystatin C) is consistently superior to eGFR based on creatinine (eGFRcreatinine) to predict these conditions.25, 26, 27, 28 The cause for the superiority of cystatin C as a risk marker is unknown, but it has been suggested that inflammation (as measured by increased C-reactive protein levels) raises the cystatin C level, thereby augmenting its potential as a risk marker.29 In elective surgery studies, however, a sharp rise in inflammation was seen in patients, with large increases in the levels of C-reactive protein and other inflammatory markers, but without any increase in the level of cystatin C.30 The relationship between raised levels of C-reactive protein and cystatin C is therefore not a causal one.
Cystatin C is a much larger molecule than creatinine (13.3kDa vs. 0.113 kDa), and this has been suggested to underlie the superiority of cystatin C as a risk factor, as kidney disease might affect the filtration of molecules differently depending on their size.4, 5 The glomerular sieving coefficient for very small molecules such as creatinine (0.113 kDa) is close to 1, but the coefficient for small proteins, for example, β-2-microglobulin (11.6 kDa), has also been described to be close to 1.31 The sieving coefficient for proteins with a size of ∼40 kDa is still more than 0.001,31 whereas very low sieving coefficients (<0.0001) have been described for proteins larger than albumin (66 kDa).31 This means that if the sieving coefficients are selectively lowered for molecules between 5 and 40 kDa, for example, increased levels of plasma proteins between 5 and 40 kDa would occur, whereas the plasma levels of molecules less than 5 kDa and more than 40 kDa would essentially be unaffected. The plasma level of a protein is determined by its production and catabolic rate, and the lower the molecular size of a protein is, the higher its catabolism by glomerular filtration will be. This means that in SPS, proteins with a molecular size like that of β-2-microglobulin or cystatin C will generally display a greater decrease in filtration, and thus in catabolism, than proteins above 40 kDa and will consequently display a higher increase in plasma level. This mechanism might explain the significant inverse correlation found in SPS (but not in reduced mGFR without SPS) between concentration change and protein size, with higher increases in the levels of smaller proteins (Figure 4). Because GFR generally is measured using molecules less than 5 kDa, measured GFR could be normal in the situation described above with selectively lowered sieving coefficients for molecules between 5 and 40 kDa, even in the presence of an abnormal ultrafiltrate and specific changes in the plasma levels of certain proteins indicating impaired filtration quality. As SPS is connected to a strong increase in mortality and morbidity, even in the absence of reduced GFR,6, 7, 8, 9 it may be important to measure “filtration quality”32 in addition to GFR when screening for kidney disease. Present screening for CKD includes eGFR, based on creatinine, cystatin C, or both, as well as the urinary albumin-to-creatinine ratio. However, this screening may be improved in the future by identifying a low eGFRcystatin C/eGFRcreatinine ratio,4, 5, 6, 9 which could signal increased risks of cardiovascular disease, end-stage renal disease, and mortality. Proteins other than cystatin C can be used to estimate GFR,21 and as they differ in molecular mass from cystatin C,21 it might be possible to characterize impaired filtration quality and its clinical consequences more carefully by using additional measurements of plasma levels of these proteins. In fact, the correlation between inflammation (raised levels of C-reactive protein) and cystatin C might reflect that inflammation generally promotes development of atherosclerosis, including in the kidneys, which might produce impaired filtration quality before it produces impaired filtration of very small molecules such as creatinine and molecules used in the measurement of GFR.
There are several previous indications that, even when mGFR is normal, abnormal glomerular filtration occurs in the third trimester of pregnancy, especially in preeclampsia. This is based not only on studies of the levels of plasma proteins,32, 33, 34, 35, 36 but also on clearance studies of dextrans of different sizes.37 Analysis of the plasma levels of proteins of different sizes in 1349 patients consecutively referred to our laboratory and with known eGFRcystatin C and eGFRcreatinine revealed that those with an eGFRcystatin C/eGFRcreatinine ratio ≤0.60 had an increase in the plasma levels of low-molecular-mass proteins similar to that observed in patients with preeclampsia.5 These results were interpreted as pointing to a common pathophysiological state of abnormal filtration quality in many types of patients other than just those with preeclampsia, and the syndrome was tentatively called “shrunken pore syndrome” to suggest a possible cause for the abnormal composition of the glomerular filtrate and the corresponding changes in the plasma levels of certain proteins.5
Although the pathophysiological mechanism in SPS might be the one discussed here, it does not directly explain the increase in mortality and morbidity connected to SPS. It is possible, however, that changes in the levels of plasma proteins resulting from impaired filtration quality might at least partly explain the increase in mortality and morbidity associated with SPS. As can be seen from the present proteomic studies, a large proportion of the changes in plasma levels of proteins in SPS or reduced GFR concerns proteins with signaling functions. A survey of the literature suggests that of the 30 changes specific for SPS, 18 promote, or are associated with, atherosclerosis38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 (Table 2). The same is true for 12 of the 31 changes occurring in patients with both SPS and reduced GFR60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 and for 10 of the 27 changes specific for reduced mGFR72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84 (Table 2). These results are compatible with the observations that the majority of the causes of morbidity and death in both patients with SPS and/or reduced GFR represent manifestations of cardiovascular disorders.6, 7, 8, 9, 14 However, the selected Olink panels used to measure protein concentrations are enriched in cardiovascular and inflammation-associated proteins. The elucidation of the exact relationships between the changes in the levels of signaling proteins and cardiovascular manifestations therefore requires extensive further studies, but our study might suggest interesting possibilities for future treatment strategies. Those proteins that play a causal role in atherosclerosis, rather than simply acting as markers for the process, might represent potential targets for therapeutic interventions to reduce the risk of cardiovascular complications, not only in SPS patients but also in all patients with reduced mGFR.
As noted in Table 1, all-cause mortality was markedly higher in patients with SPS, both in patients with normal and in patients with reduced GFR. Differences between the groups with respect to age, gender, and BMI were small and could not explain the association between SPS and mortality. We thus hypothesize that the difference in mortality is due to SPS, but we cannot rule out the possibility that the difference is confounded by other CV risk factors that were not available for the present investigation. In ongoing work, we plan to synthesize and analyze more detailed register data for the full LCS cohort of 2805 patients,11 also with respect to cause-specific mortality.
Disclosure
MSA, ASV, ÖL and GF are employed at Olink Proteomics AB, Uppsala, Sweden, as of May 2018. All the other authors declared no competing interests.
Acknowledgments
This investigation is dedicated to the late Bengt Rippe for his ground-breaking work on the pore model of capillary permeability. The study was supported by grants from the Alfred Österlund Foundation, the Medical Faculty of the University of Lund, and from Region Skåne.
Footnotes
Table S1. Statistical analysis of the concentration changes of 177 plasma proteins in samples from 156 patients without or with shrunken pore syndrome (SPS) and with measured normal or reduced glomerular filtration rate (rGFR) to establish changes specific for SPS and rGFR.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Statistical analysis of the concentration changes of 177 plasma proteins in samples from 156 patients without or with shrunken pore syndrome (SPS) and with measured normal or reduced glomerular filtration rate (rGFR) to establish changes specific for SPS and rGFR.
References
- 1.Shlipak M.G., Fried L.F., Cushman M. Cardiovascular mortality risk in chronic kidney disease. Comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 2.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest. 2010;70:65–70. doi: 10.3109/00365511003642535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubb A., Nyman U., Björk J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest. 2012;72:73–77. doi: 10.3109/00365513.2011.634023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb A. Cystatin C as a multifaceted biomarker in kidney disease and its role in defining “shrunken pore syndrome.”. In: Edelstein C.L., editor. Biomarkers in Kidney Disease. Elsevier; Atlanta, GA: 2017. pp. 225–240. [Google Scholar]
- 5.Grubb A., Lindström V., Jonsson M. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: “shrunken pore syndrome”. Scand J Clin Lab Invest. 2015;75:333–340. doi: 10.3109/00365513.2015.1025427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dardashti A., Nozohoor S., Grubb A. Shrunken pore syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76:74–81. doi: 10.3109/00365513.2015.1099724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purde M.T., Nock S., Risch L. The cystatin C/creatinine ratio, a marker of glomerular filtration quality: associated factors, reference intervals, and prediction of morbidity and mortality in healthy seniors. Transl Res. 2016;169:80–90. doi: 10.1016/j.trsl.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Purde M.T., Nock S., Risch L. Ratio of cystatin C and creatinine-based estimates of the glomerular filtration rate predicts mortality in healthy seniors independent of kidney function. Scand J Clin Lab Invest. 2016;76:341–343. doi: 10.3109/00365513.2016.1149882. [DOI] [PubMed] [Google Scholar]
- 9.Christensson A., Grubb A., Molvin J. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population—the HARVEST study. Scand J Clin Lab Invest. 2016;76:568–574. doi: 10.1080/00365513.2016.1223338. [DOI] [PubMed] [Google Scholar]
- 10.Sundin P.O., Sjöström P., Jones I. Measured glomerular filtration rate does not improve prediction of mortality by cystatin C and creatinine. Nephrol Dial Transplant. 2017;32:663–670. doi: 10.1093/ndt/gfx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grubb A., Horio M., Hansson L.O. Generation of a new cystatin C-based estimating equation for glomerular filtration rate using seven assays standardized to the international calibrator. Clin Chem. 2014;60:974–986. doi: 10.1373/clinchem.2013.220707. [DOI] [PubMed] [Google Scholar]
- 12.Krutzén E., Bäck S.E., Nilsson-Ehle I. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955–961. [PubMed] [Google Scholar]
- 13.Soveri I., Berg U.B., Björk J. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Assarsson E., Lundberg M., Holmquist G. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyhse-Andersen J., Schmidt C., Nordin G. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 16.Grubb A., Blirup-Jensen S., Lindström V., on behalf of the IFCC Working Group on standardisation of cystatin C (WG-SCC) First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 17.Björk J., Grubb A., Larsson A. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: a cross-sectional study in Sweden. Clin Chem Lab Med. 2015;53:403–414. doi: 10.1515/cclm-2014-0578. [DOI] [PubMed] [Google Scholar]
- 18.Nyman U., Grubb A., Larsson A. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52:815–824. doi: 10.1515/cclm-2013-0741. [DOI] [PubMed] [Google Scholar]
- 19.Risch M., Risch L., Purde M.T. Association of the cystatin C/creatinine ratio with the renally cleared hormones parathyroid hormone (PTH) and brain natriuretic peptide (BNP) in primary care patients: a cross-sectional study. Scand J Clin Lab Invest. 2016;76:379–385. doi: 10.1080/00365513.2016.1183262. [DOI] [PubMed] [Google Scholar]
- 20.Löfberg H., Grubb A. Quantitation of gamma-trace in human biological fluids: indications for production in the central nervous system. Scand J Clin Lab Invest. 1979;39:619–626. doi: 10.3109/00365517909108866. [DOI] [PubMed] [Google Scholar]
- 21.Grubb A., Simonsen O., Sturfelt G. Serum concentration of cystatin C, factor D and beta-2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 22.Dharnidharka V.R., Kwon C., Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 23.Grubb A. Cystatin C is indispensable for evaluation of kidney disease. EJIFCC. 2017;28:269–276. [PMC free article] [PubMed] [Google Scholar]
- 24.Christensson A., Ash J.A., DeLisle R.K. The impact of the glomerular filtration rate on the human plasma proteome. Proteomics Clin Appl. 2018;12:e1700067. doi: 10.1002/prca.201700067. [DOI] [PubMed] [Google Scholar]
- 25.Jernberg T., Lindahl B., James S. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- 26.Shlipak M.G., Sarnak M.J., Katz R. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 27.Peralta C.A., Shlipak M.G., Judd S. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak M.G., Matsushita K., Ärnlöv J. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight E.L., Verhave J.C., Spiegelman D. Factors influencing cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 30.Grubb A., Björk J., Nyman U. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest. 2011;71:145–149. doi: 10.3109/00365513.2010.546879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norden A.G.W., Lapsley M., Lee P.J. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001;60:1885–1892. doi: 10.1046/j.1523-1755.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 32.Grubb A., Lindström V., Kristensen K. Filtration quality: a new measure of renal disease. Clin Chem Lab Med. 2007;45(suppl):S273–S274. [Google Scholar]
- 33.Strevens H., Wide-Swensson D., Torffvit O., Grubb A. Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women. Indications of altered filtration process in pregnancy. Scand J Clin Lab Invest. 2002;62:141–147. doi: 10.1080/003655102753611771. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen K., Lindström V., Schmidt C. Temporal changes of the plasma levels of cystatin C, beta-trace protein, beta-2-microglobulin, urate and creatinine during pregnancy indicate continuous alterations in the renal filtration process. Scand J Clin Lab Invest. 2007;67:612–618. doi: 10.1080/00365510701203488. [DOI] [PubMed] [Google Scholar]
- 35.Strevens H., Wide-Swensson D., Grubb A. Serum cystatin C is a better marker for preeclampsia than serum creatinine or serum urate. Scand J Clin Lab Invest. 2001;61:575–580. doi: 10.1080/003655101753218346. [DOI] [PubMed] [Google Scholar]
- 36.Kristensen K., Wide-Swensson D., Schmidt C. Cystatin C, beta-2-microglobulin and beta-trace protein in pre-eclampsia. Acta Obstet Gynecol Scand. 2007;86:921–926. doi: 10.1080/00016340701318133. [DOI] [PubMed] [Google Scholar]
- 37.Roberts M., Lindheimer M.D., Davison J.M. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol. 1996;270:F338–F343. doi: 10.1152/ajprenal.1996.270.2.F338. [DOI] [PubMed] [Google Scholar]
- 38.Maddaluno M., Di Lauro M., Di Pascale A. Monocyte chemotactic protein-3 induces human coronary smooth muscle cell proliferation. Atherosclerosis. 2011;217:113–119. doi: 10.1016/j.atherosclerosis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Jin S.Y., Tohyama J., Bauer R.C. Genetic ablation of Adamts13 gene dramatically accelerates the formation of early atherosclerosis in a murine model. Arterioscler Thromb Vasc Biol. 2012;32:1817–1823. doi: 10.1161/ATVBAHA.112.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiechl S., Schett G., Wenning G. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 41.Dewberry R., Holden H., Crossman D., Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;11:2394–2400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 42.Hartman J., Frishman W.H. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;3:147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 43.Schieffer B., Selle T., Hilfiker A. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 44.Butcher M.J., Waseem T.C., Galkina E.V. Smooth muscle cell-derived interleukin-17C plays an atherogenic role via the recruitment of proinflammatory interleukin-17A+ T cells to the aorta. Arterioscler Thromb Vasc Biol. 2016;36:1496–1506. doi: 10.1161/ATVBAHA.116.307892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelken N.A., Coughlin S.R., Gordon D. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;4:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedjai B., Li H., Stroke I.L. Small molecule chemokine mimetics suggest a molecular basis for the observation that CXCL10 and CXCL11 are allosteric ligands of CXCR3. Br J Pharmacol. 2012;166:912–923. doi: 10.1111/j.1476-5381.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jefferis B.J., Papacosta O., Owen C.G. Interleukin 18 and coronary heart disease: prospective study and systematic review. Atherosclerosis. 2011;217:227–233. doi: 10.1016/j.atherosclerosis.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhavanpoor M., Gleissner C.A., Gorbatsch S. CCL19 and CCL21 modulate the inflammatory milieu in atherosclerotic lesions. Drug Des Devel Ther. 2014;8:2359–2371. doi: 10.2147/DDDT.S72394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Sun C., Gerdes N. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med. 2015;7:820–826. doi: 10.1038/nm.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi B., Du X., Wang Q. Increased PD-1 on CD4(+)CD28(−) T cell and soluble PD-1 ligand-1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metabolism. 2013;62:778–785. doi: 10.1016/j.metabol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Konya H., Miuchi M., Satani K. Hepatocyte growth factor, a biomarker of macroangiopathy in diabetes mellitus. World J Diabetes. 2014;5:678–688. doi: 10.4239/wjd.v5.i5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawamoto R., Oka Y., Yoshida O. Significance of serum circulating hepatocyte growth factor in the development of carotid atherosclerosis. J Atheroscler Thromb. 2003;10:154–159. doi: 10.5551/jat.10.154. [DOI] [PubMed] [Google Scholar]
- 53.Fornai F., Carrizzo A., Forte M. The inflammatory protein pentraxin 3 in cardiovascular disease. Immun Ageing. 2016;13:25–34. doi: 10.1186/s12979-016-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Borne P, Quax PH, Hoefer IE, et al. The multifaceted functions of CXCL10 in cardiovascular disease [e-pub ahead of print]. Biomed Res Int. https://doi.org/10.1155/2014/893106, Accessed October 25, 2018. [DOI] [PMC free article] [PubMed]
- 55.Heller E.A., Liu E., Tager A.M. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 56.Duncan R.F., Peterson H., Hagedorn C.H., Sevanian A. Oxidative stress increases eukaryotic initiation factor 4E phosphorylation in vascular cells. Biochem. J. 2003;369:213–225. doi: 10.1042/BJ20020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Kornmark L., Jonasson L. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2009;202:92–102. doi: 10.1016/j.atherosclerosis.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Calvayrac O., Rodríguez-Calvo R., Alonso J. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells. Role of NF- kB. Arterioscler Thromb Vasc Biol. 2011;31:2733–2741. doi: 10.1161/ATVBAHA.111.235721. [DOI] [PubMed] [Google Scholar]
- 59.Wan W., Murphy P.M. Regulation of atherogenesis by chemokine receptor CCR6. Trends Cardiovasc Med. 2011;21:140–144. doi: 10.1016/j.tcm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beygui F., Wild P.S., Zeller T. Adrenomedullin and arterial stiffness: integrative approach combining monocyte ADM expression, plasma MR-Pro-ADM, and genome-wide association study. Circ Cardiovasc Genet. 2014;7:634–641. doi: 10.1161/CIRCGENETICS.113.000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rambod M., Heine G.H., Seiler S. Association of vascular endothelial factors with cardiovascular outcome and mortality in chronic kidney disease patients: a 4-year cohort study. Atherosclerosis. 2014;236:360–365. doi: 10.1016/j.atherosclerosis.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skau E., Henriksen E., Wagner P. GDF-15 and TRAIL-R2 are powerful predictors of long-term mortality in patients with acute myocardial infarction. Eur J Prev Cardiol. 2017;24:1576–1583. doi: 10.1177/2047487317725017. [DOI] [PubMed] [Google Scholar]
- 63.Jin W, Zhao Y, Yan W, et al. Elevated circulating interleukin-27 in patients with coronary artery disease is associated with dendritic cells, oxidized low-density lipoprotein, and severity of coronary artery stenosis [e-pub ahead of print]. Mediators Inflamm. https://doi.org/10.1155/2012/506283, Accessed October 25, 2018. [DOI] [PMC free article] [PubMed]
- 64.Gonçalves I., Edsfeldt A., Colhoun H.M. Association between renin and atherosclerotic burden in subjects with and without type 2 diabetes. BMC Cardiovasc Disord. 2016;16:171. doi: 10.1186/s12872-016-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahran M., Nasr F.M., Metwaly A.A. The role of hemostatic factors in atherosclerosis in patients with chronic renal disease. Electron Physician. 2015;7:1270–1276. doi: 10.14661/1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Aguilar E., Gomez-Rodriguez V., Orbe J. Matrix metalloproteinase 10 is associated with disease severity and mortality in patients with peripheral arterial disease. J Vasc Surg. 2015;61:428–435. doi: 10.1016/j.jvs.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Kim C.S., Kang J.H., Cho H.R. Potential involvement of CCL23 in atherosclerotic lesion formation/progression by the enhancement of chemotaxis, adhesion molecule expression, and MMP-2 release from monocytes. Inflamm Res. 2011;60:889–895. doi: 10.1007/s00011-011-0350-5. [DOI] [PubMed] [Google Scholar]
- 68.Lievens D., Eijgelaar W.J., Biessen E.A. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb Haemost. 2009;102:206–214. doi: 10.1160/TH09-01-0029. [DOI] [PubMed] [Google Scholar]
- 69.Stolla M., Pelisek J., von Brühl M.L. Fractalkine is expressed in early and advanced atherosclerotic lesions and supports monocyte recruitment via CX3CR1. PLoS One. 2012;7:e43572. doi: 10.1371/journal.pone.0043572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goettsch C., Rauner M., Sinningen K. The osteoclast-associated receptor (OSCAR) is a novel receptor regulated by oxidized low-density lipoprotein in human endothelial cells. Endocrinology. 2011;152:4915–4926. doi: 10.1210/en.2011-1282. [DOI] [PubMed] [Google Scholar]
- 71.Söderström L.Å., Gertow K., Folkersen L. Human genetic evidence for involvement of CD137 in atherosclerosis. Mol Med. 2014;20:456–465. doi: 10.2119/molmed.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steffel J., Lüscher T.F., Tanner F.C. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 73.Wannamethee S.G., Welsh P., Papacosta O. Circulating soluble receptor for advanced glycation end product: cross-sectional associations with cardiac markers and subclinical vascular disease in older men with and without diabetes. Atherosclerosis. 2017;264:36–43. doi: 10.1016/j.atherosclerosis.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kendrick J., Cheung A.K., Kaufman J.S. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munoz Mendoza J., Isakova T., Cai X. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017;91:711–719. doi: 10.1016/j.kint.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erbel C., Wolf A., Lasitschka F. Prevalence of M4 macrophages within human coronary atherosclerotic plaques is associated with features of plaque instability. Int J Cardiol. 2015;186:219–225. doi: 10.1016/j.ijcard.2015.03.151. [DOI] [PubMed] [Google Scholar]
- 77.Erbel C., Tyka M., Helmes C.M. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015;21:255–265. doi: 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- 78.LaFramboise W.A., Dhir R., Kelly L.A. Serum protein profiles predict coronary artery disease in symptomatic patients referred for coronary angiography. BMC Med. 2012;10:157. doi: 10.1186/1741-7015-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi J., Mabuchi H. Lipoprotein lipase and atherosclerosis. Ann Clin Biochem. 2015;52:632–637. doi: 10.1177/0004563215590451. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto S., Kishida K., Shimomura I. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun. 2002;292:781–786. doi: 10.1006/bbrc.2002.6720. [DOI] [PubMed] [Google Scholar]
- 81.Kalay N., Yarlioglues M., Ardic I. The role of heart-type fatty acid-binding protein in predicting properties of coronary atherosclerosis in patients with acute coronary syndrome. Coron Artery Dis. 2010;21:435–440. doi: 10.1097/MCA.0b013e32833db539. [DOI] [PubMed] [Google Scholar]
- 82.Abd Alla J., Langer A., Elzahwy S.S. Angiotensin-converting enzyme inhibition down-regulates the pro-atherogenic chemokine receptor 9 (CCR9)-chemokine ligand 25 (CCL25) axis. J Biol Chem. 2010;285:23496–23505. doi: 10.1074/jbc.M110.117481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Wong B.W., Wong D., Luo H. Vascular endothelial growth factor-D is overexpressed in human cardiac allograft vasculopathy and diabetic atherosclerosis and induces endothelial permeability to low-density lipoproteins in vitro. J Heart Lung Transplant. 2011;30:955–962. doi: 10.1016/j.healun.2011.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis of the concentration changes of 177 plasma proteins in samples from 156 patients without or with shrunken pore syndrome (SPS) and with measured normal or reduced glomerular filtration rate (rGFR) to establish changes specific for SPS and rGFR.