Figure 1.
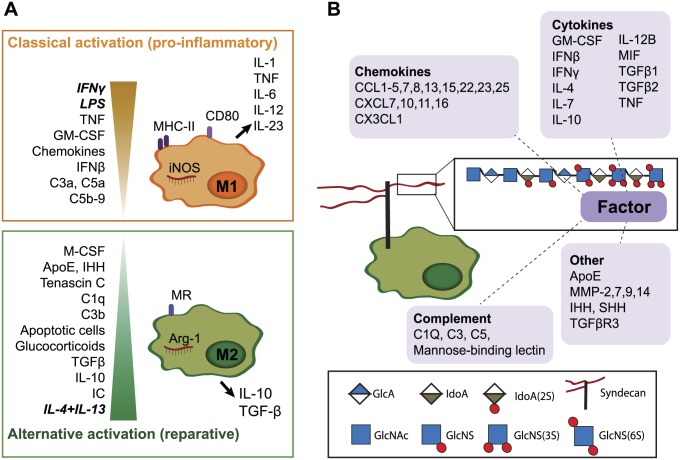
Several macrophage-modulating bioactive proteins bind to HS. (A). In vitro, two extreme activation signatures are defined for macrophages. Interferon-γ (IFNγ) and lipopolysaccharide (LPS) or tumor necrosis factor (TNF) are responsible for classical activation (previously termed “M1”), which is characterized by nitric oxide synthase (iNOS), cluster of differentiation 80 (CD80) and major histocompatibility complex-II (MHC-II) expression and secretion of pro-inflammatory cytokines. IL-4 and IL-13 result in alternative macrophage activation (previously termed “M2”), which is associated with arginase (Arg-1) and mannose receptor (MR or CD206) expression and secretion of IL-10 and TGFβ. In vivo, heterogeneous activation states are present, depending on stimuli in their local environment.5,6 Granulocyte-macrophage colony-stimulating factor (GM-CSF), various chemokines7 and the complement factors C3a, C5a, and C5b-98 also promote M1-like differentiation, while immune complexes (IC), IL-10, TGFβ, glucocorticoids,6 the complement factors C1q and C3b,8 tenascin C,9 Indian hedgehog (IHH) signaling,10 and apolipoprotein E (ApoE)11 contribute to M2-like activation. Many additional factors are also likely to affect macrophage polarization. (B). Many HS-binding proteins (as reviewed by Ori et al.12) are known to modulate macrophage function. In addition to the well-known cytokines and chemokines,7 proteins such as complement factors can modulate macrophage activation.8 MMPs are involved in macrophage infiltration13–15 and MMP-7 suppress M1 polarization, while SHH has been shown to act as a macrophage chemoattractant.16 Abbreviations: HS, heparan sulfate; TGFβ, transforming growth factor beta; IL, interleukin; MMPs, matrix metalloproteinases.