Abstract
Islet microvasculature provides key architectural and functional roles, yet the morphological features of islets from patients with type 1 diabetes are poorly defined. We examined islet and exocrine microvasculature networks by multiplex immunofluorescence imaging of pancreases from organ donors with and without type 1 diabetes (n=17 and n=16, respectively) and determined vessel diameter, density, and area. We also analyzed these variables in insulin-positive and insulin-negative islets of 7 type 1 diabetes donors. Control islet vessel diameter was significantly larger (7.6 ± 1.1 μm) compared with vessels in diabetic islets (6.2 ± 0.8 μm; p<0.001). Control islet vessel density (number/islet) was significantly lower (5.3 ± 0.6) versus diabetic islets (9.3 ± 0.2; p<0.001). Exocrine vessel variables were not significantly different between groups. Islets with residual beta-cells were comparable to control islets for both vessel diameter and density and were significantly different from insulin-negative islets within diabetic donors (p<0.05). Islet smooth muscle actin area had a significant positive correlation with age in both groups (p<0.05), which could negatively impact islet transplantation efficiency from older donors. These data underscore the critical relationship of islet beta-cells and islet vessel morphology in type 1 diabetes. These studies provide new knowledge of the islet microvasculature in diabetes and aging.
Keywords: alpha-cell, CD3, CD31, CD34, endothelium, exocrine, glucagon, insulin, microenvironment, secretogranin 3, smooth muscle actin
Introduction
Type 1 diabetes is a chronic disorder resulting from complex genetic, environmental, and immunological factors that drive autoimmune responses to islet beta-cell antigens resulting in the irreversible loss of beta-cell mass.1,2 These destructive processes begin months to years before clinical (i.e., symptomatic) onset of the disease. Ongoing autoimmunity and beta-cell dysfunction and destruction are asymptomatic during this prodromal period but can be identified serologically by the presence of autoantibodies against one or more beta-cell autoantigens and metabolically by the loss of first-phase insulin secretion in response to glucose.3,4 After clinical onset, a partial remission phase (so-called “honeymoon”) can occur where the patient may require less insulin to maintain glycemic control.5,6 This phase has been hypothesized to represent a critical window for inducing adult beta-cell regeneration and/or replacement in conjunction with anti-autoimmune strategies to prevent further beta-cell losses. An improved understanding of the pathological processes occurring within islets containing residual beta-cells would aid in the design of therapeutic strategies after onset of diabetes.
The islet microvasculature is organized into a glomerular-like network of highly fenestrated capillaries surrounded by a unique double basement membrane.7–13 The islet microvasculature is required for normal endocrine cell development and serves a critical role in hormone secretion.11,14,15 Pancreatic islets are well perfused, with an estimated blood perfusion rate of 5–15% of the entire organ despite representing only 1–2% of pancreatic volume.9,14,16 Each islet endocrine cell is in close proximity to the microvasculature, ensuring efficient transfer of nutrients and signaling factors between endocrine and endothelial cells.17–19 Islet blood flow rate is regulated through complex interactions between the autonomic and sensory nervous systems and paracrine factors from endocrine cells.7,9 The microvasculature also serves an important barrier function to leukocyte transmigration, and loss of endothelial integrity contributes to inflammation.14
Recent studies show that the human islet microvasculature is ~5-fold less dense (number of vessels/islet area) than in mouse islets.10,20–23 Human islet vessels were reported to contain three-times higher α-smooth muscle actin (SMA) cells compared with islets from mice where SMA cells were found associated only with feeding arterioles found at the islet periphery.21 Furthermore, patients with type 2 diabetes had increased islet vessel density or area compared with non-diabetic donors.20,23 The purpose of the current study was to conduct morphometric studies of the islet microvasculature in patients with type 1 diabetes in comparison to matched control donors. Both islet and exocrine microvascular networks were analyzed for vessel diameters, densities, and areas and SMA areas. Islet vessel morphology was also studied in a subset of type 1 diabetes patients with residual beta-cells and another subset of those donors with insulitis in insulin-positive islets.
Materials and Methods
Donors and Pancreas Samples
Formalin-fixed, paraffin-embedded serial sections were provided by the JDRF Network for Pancreatic Organ donors with Diabetes (nPOD) program using protocols previously described for pancreas recovery and immersion fixation.24 Donors included (1) non-diabetic controls with no history of diabetes or pancreatic disease and negative for type 1 diabetes-associated autoantibodies (control, n=16), and (2) donors with type 1 diabetes (n=17; Table 1). These studies were conducted following approval by the University of Florida Institutional Review Board (521-2008) under an exempt status for cadaveric studies. Serial paraffin sections (4 μm) were obtained from blocks in the body-tail region previously screened by H&E and IHC.25
Table 1.
Donor Demographics and Beta-cell Analyses.
| Case ID | Age (Years) | Diabetes Duration (Years) | Sex | Race | BMI | C-Pepa | Cause of Death | AAbb | Insulin Area (%)c | Insulin Mass (mg)c |
|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes (Control) | ||||||||||
| 6318 | 10.0 | F | Caucasian | 17.6 | 3.89 | Head Trauma | Negative | 0.87 | 262 | |
| 6358 | 13.0 | M | African American | 19.2 | 8.00 | Cerebrovascular/Stroke | Negative | 1.16 | 609 | |
| 6233 | 14.0 | M | Caucasian | 21.9 | 7.26 | Anoxia | Negative | 2.62 | 1596 | |
| 6282 | 14.0 | M | Caucasian | 41.9 | 6.83 | Head Trauma | Negative | 3.81 | 2890 | |
| 6232 | 14.0 | F | Caucasian | 20.8 | 19.50 | Head Trauma | Negative | 1.79 | 886 | |
| 6099 | 14.2 | M | Caucasian | 30.0 | 5.37 | Head Trauma | Negative | 1.53 | 1307 | |
| 6317 | 15.0 | M | Caucasian | 29.8 | 7.15 | Head Trauma | Negative | 1.79 | 796 | |
| 6271 | 17.0 | M | Caucasian | 24.4 | 11.47 | Head Trauma | Negative | 1.99 | 1950 | |
| 6227 | 17.0 | F | Caucasian | 26.4 | 2.75 | Cerebrovascular/Stroke | Negative | 1.21 | 731 | |
| 6279 | 19.0 | M | Caucasian | 34.0 | 8.01 | Head Trauma | Negative | 2.79 | 2237 | |
| 6238 | 20.0 | M | African American | 21.7 | 1.17 | Head Trauma | Negative | 1.83 | 1675 | |
| 6179 | 21.8 | F | Caucasian | 20.7 | 2.74 | Head Trauma | Negative | NA | NA | |
| 6057 | 22.0 | M | Caucasian | 26.0 | 16.23 | Head Trauma | Negative | 1.59 | 1657 | |
| 6160 | 22.1 | M | Caucasian | 23.9 | 0.40 | Head Trauma | Negative | 0.51 | 403 | |
| 6333 | 27.0 | F | Caucasian | 24.9 | 9.37 | Anoxia | Negative | 0.66 | 453 | |
| 6091 | 27.1 | M | Caucasian | 35.6 | 7.71 | Head Trauma | Negative | 0.69 | 658 | |
| Mean ± SD | 18.0 ± 5.0 | 5F/11M | 26.2 ± 6.6 | 7.37 ± 5.1 | 1.66 ± 0.90 | 1207 ± 770 | ||||
| Type 1 Diabetes | ||||||||||
| 6265 | 11.0 | 8 | M | Caucasian | 12.9 | 0.06 | Cerebrovascular/Stroke | GADA+ mIAA+ | 0.06 | 10 |
| 6264 | 12.0 | 9 | F | Caucasian | 22.0 | 0.00 | DKA, cerebral edema | Negative | 0.06 | 13 |
| 6243 | 13.0 | 5 | M | Caucasian | 21.3 | 0.42 | Cerebrovascular/Stroke | mIAA+ | 0.50 | 147 |
| 6089 | 14.3 | 8 | M | Caucasian | 26.0 | 0.00 | Anoxia | mIAA+ | 0.00 | 1 |
| 6049 | 15.0 | 10 | F | African American | 20.8 | 0.00 | Anoxia | GADA+ mIAA+ | 0.05 | 9 |
| 6083 | 15.2 | 11 | F | Caucasian | 18.4 | 0.00 | DKA, cerebral edema | mIAA+ | 0.00 | 1 |
| 6396 | 17.1 | 2 | F | Caucasian | 22.6 | 0.06 | DKA, cerebral edema | Negative | 0.01 | 4 |
| 6237 | 18.0 | 12 | F | Caucasian | 26.0 | 0.00 | Head Trauma | GADA+ mIAA+ | 0.01 | 3 |
| 6046 | 18.8 | 8 | F | Caucasian | 25.2 | 0.00 | Anoxia | IA-2A+ ZnT8A+ | 0.05 | 17 |
| 6195 | 19.3 | 5 | M | Caucasian | 23.7 | 0.00 | Head Trauma | GADA+ IA-2A+ ZnT8A+ mIAA+ | 0.02 | 6 |
| 6064 | 19.6 | 9 | F | Caucasian | 22.6 | 0.00 | Anoxia | GADA+ IA-2A+ ZnT8A+ mIAA+ | 0.01 | 5 |
| 6325 | 20.0 | 6 | F | African American | 31.2 | 0.14 | Anoxia | GADA+ IA-2A+ mIAA+ | 0.31 | 129 |
| 6245 | 22.0 | 7 | M | Caucasian | 23.2 | 0.00 | Head Trauma | GADA+ IA-2A+ | 0.55 | 174 |
| 6070 | 22.6 | 7 | F | Caucasian | 21.6 | 0.00 | Anoxia | IA-2A+ mIAA+ | 0.09 | 36 |
| 6362 | 24.9 | 0 | M | Caucasian | 28.5 | 0.38 | Head Trauma | GADA+ | 0.33 | 176 |
| 6321 | 27.0 | 16 | F | Caucasian | 20.3 | 0.00 | Anoxia | IA-2A+ ZnT8A+ mIAA+ | 0.00 | 0 |
| 6324 | 29.0 | 2 | M | Hispanic | 26.2 | 0.00 | Anoxia | GADA+ mIAA+ | 0.00 | 0 |
| Mean ± SD | 18.8 ± 5.2 | 7.4 ± 3.9 | 10F/7M | 23.1 ± 4.1 | 0.06 ± 0.13 | 0.12 ± 0.18 | 43 ± 66 | |||
Abbreviations: BMI, body mass index; DKA, diabetic ketoacidosis; GADA, glutamic acid decarboxylase; mIAA, insulin; IA-2A, insulinoma-associated protein-2; ZnT8A, zinc transporter-8; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases.
C-peptide levels (μg/ml).
Autoantibody (AAb) status. All donors had AAb testing by radioimmunoassay (RIA) as previously reported.26 RIA data values were converted to NIDDK units and defined as positive if one or more of the following applied: GADA if ≥20, IA-2A if ≥5, mIAA+ if ≥ 0.10, or ZnT8A if ≥0.020. mIAA+ antibodies may present those resulting from insulin therapy.
Insulin fractional area and mass were previously reported.27
Multiplex Immunofluorescence
Multiplex immunofluorescence was conducted using 4 primary antibodies to detect all neuroendocrine cells: secretogranin 3 (SCG3), beta-cells (insulin), vascular endothelium (CD34, CD31), and SMA following methods previously described27 (Table 2). Control stains showed that islet vessels were uniformly labeled by both CD34 and CD31 (Supplemental Fig. 1). Paraffin sections were dewaxed and rehydrated with TBS and with 0.05% TBST. Nonspecific binding was blocked by incubation in 10% goat serum in TBST for 1 hr at room temperature. Sections were incubated in primary antibodies to SCG3 for 1 hr at room temperature followed by tyramine signal amplification (TSA) with Opal 520 (PerkinElmer; Waltham, MA) conjugate followed by anti-CD34 for 1 hr at room temperature and TSA with Opal 670 conjugate. Sections were reblocked before incubation with anti-insulin antibody overnight at 4C, followed by TBST washes and incubation with goat antiguinea pig Alexa 405 (ThermoFisher Scientific; Waltham, MA) for 1 hr at room temperature. Tissues were reblocked and mouse anti-SMA-Cy3 antibody applied overnight at 4C. Sections were mounted with ProLong Gold Antifade (Life Technologies; Grand Island, NY). Additional sections were stained in 4 control donors and 8 donors with type 1 diabetes for SCG3, CD3, glucagon, and insulin and insulitis determined as previously described.27
Table 2.
Antibodies Used for the Analysis of the Human Pancreas Microvasculature.
| Antigen | Target | Host | Company | Catalog No. | Dilution |
|---|---|---|---|---|---|
| SMA | Smooth muscle cells | Mouse | Sigma; St. Louis, MO | 6198 | 1:200 |
| CD3 | T Lymphocytes | Rabbit | Dako; Carpinteria, CA | A0452 | 1:500 |
| CD31 | Endothelial cells | Rabbit | Abcam; Cambridge, MA | AB28364 | 1:250 |
| CD34 | Endothelial cells | Mouse | Life Tech.; Grand Island, NY | MA1-80202 | 1:300 |
| GCG | Alpha-cells | Mouse | Abcam | AB10988 | 1:200 |
| INS | Beta-cells | Guinea Pig | Dako | A0564 | 1:100 |
| SCG3 | Neuroendocrine cells | Rabbit | Sigma | HPA006880 | 1:1000 |
Secondary antibodies were Opal (PerkinElmer; Waltham, MA) or AlexaFluor (ThermoFisher Scientific; Waltham, MA) conjugates at 1:200–1:1000 dilutions. Abbreviations: SMA, α-Smooth Muscle Actin-Cy3; CD3/31/34, vascular endothelium; GCG, glucagon; INS, insulin; SCG3, secretogranin 3.
Confocal Microscopy
Photomicroscopy was performed on a Zeiss 710 LSM confocal microscope using the 405, 488, 561, and 633 nm laser lines to excite AlexaFluor 405, Opal 520, Cy3, and Opal 670, respectively. Opal 520 and 670 emissions were collected simultaneously (510–545 nm and 640–750 nm) whereas AlexaFluor 405 (405–470 nm) and Cy3 (560–595 nm) were collected separately to eliminate fluorochrome crosstalk. Sections were photographed and analyzed by one observer (J.C.). The entire pancreas section was scanned with a 10× objective, then islets were randomly selected from multiple lobules after careful evaluation of the entire section using the selection criteria as follows: (1) spherical to oval shape, (2) diameter between ~50–150 μm, and (3) no immediately adjacent duct or large artery. Islets were imaged using a Plan-Apochromat 20× objective (0.8 numerical aperture, 0.15 μm/pixel), maximum resolution, and 1 airy unit pinhole. Numbers of islets and peri-islet regions analyzed per donor are shown in Supplemental Table 1. For the donors with type 1 diabetes, additional islets were imaged with residual beta-cells (INS+, n=7 donors). INS+ islets were infrequent and often found within certain lobules and, hence, could not be randomly sampled (Supplemental Table 1). Insulitic islets were further studied in the INS+ islets (n=5 donors). Insulitic islets were defined as 3 or more islets per section with >5 CD3+ cells/islet, immediately adjacent to or within INS+ islets as used in a previous study.27
Morphometric Analyses
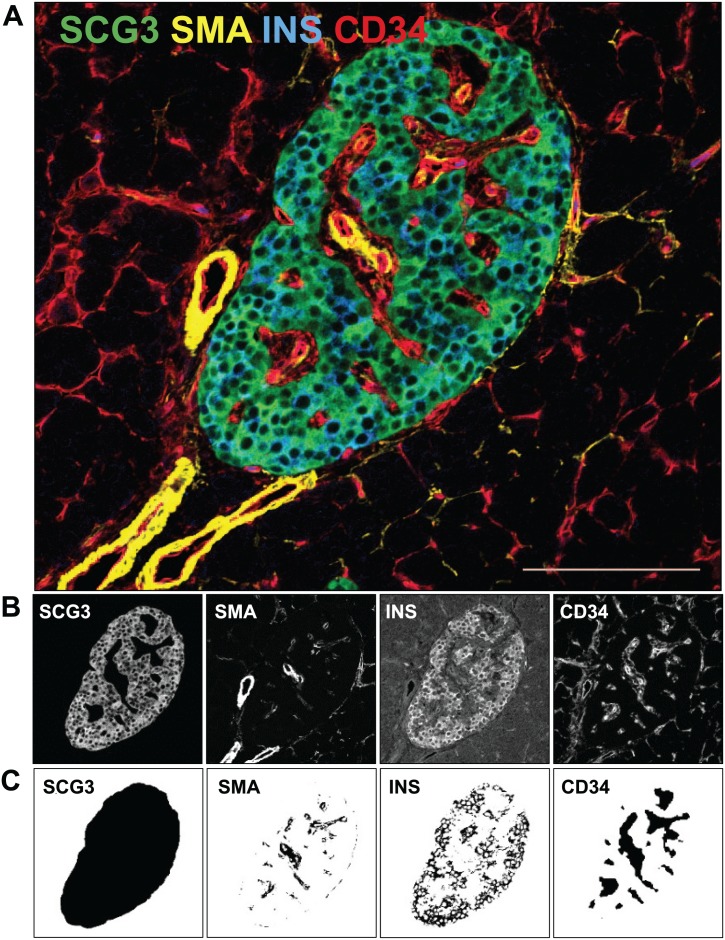
Endocrine and exocrine vessel morphometric analyses were performed using custom Python scripts created for FIJI software (v2.0; https://fiji.sc).28 Total islet area was identified by thresholding the SCG3+ area and applying morphological filters to acquire a smoothed islet boundary to include all SCG3+, CD34+, and SMA+ areas (Fig. 1). Islets were categorized for insulin immunopositivity (INS+/–) using a background-corrected insulin intensity and visual confirmation for every islet. Islet CD34+ area (%), insulin area (%), and SMA+ area (%) were determined via thresholding of the immunostained area divided by total islet area. Particle analysis was used to determine CD34+ islet vessel density (vessels/μm2 islet area) based on previously described methods for individual vessel counts and area (%).29 The plug-in Geodesic Diameters was used to determine vessel diameters via maximum inscribed circles for each vessel, and average vessel diameter was determined for each islet.30 Similar calculations were performed in the same image field for the peri-islet exocrine microvasculature excluding islets and major vessels and ducts.
Figure 1.
Image analysis procedure. A representative merged islet image is shown from a 20-year-old male African American donor without diabetes (A). Sections were stained for SCG3 (green, total islet), SMA (yellow, smooth muscle cells), insulin (blue, beta-cells), and CD34 (red, vessels) as described in section “Materials and Methods.” Image analysis segmentation steps for each stain are shown before (B) and after (C) each processing step. Scale bar = 100 µm. Abbreviations: SCG3, secretogranin 3; SMA, smooth muscle actin; INS, insulin; CD34, vascular endothelium.
Statistics
Data are expressed as means ± SD with number of donors (N) unless otherwise noted, using 5–35 islets/donor. Unpaired Mann-Whitney U tests were used to compare islet and peri-islet exocrine vessels between control and diabetic donor groups. Wilcoxon matched-pairs signed rank tests were used to compare islet and exocrine vessels within diabetic donors for islet INS+/– and CD3+/– subtypes. All data were compiled in Excel, and statistics were performed using GraphPad Prism v6 (GraphPad Software Inc.; San Diego, CA). Correlations were tested by Spearman’s analysis with linear regression analysis. A p<0.05 was considered statistically significant.
Results
Islet Vessels Were Smaller and More Numerous in Donors With Type 1 Diabetes
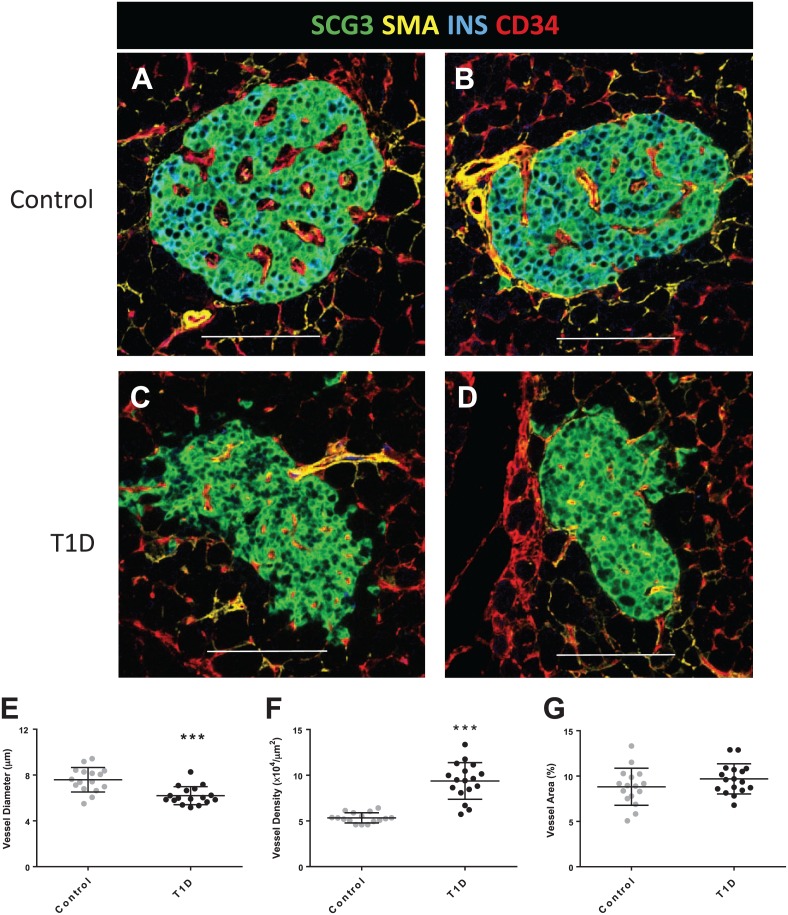
The marker SCG3 labeled the cytoplasmic compartment in all islet neuroendocrine cells and delineated total islet area using the custom script (Fig. 1A–C; SCG3). In islets, SMA+ areas were found along most CD34+ areas with the greatest density found in larger feeding arterioles at the periphery of the islets that extended inward for variable distances (Fig. 1A–C; SMA). Within the exocrine regions surrounding islets, SMA+ cells were observed primarily at larger intralobular arterioles (not shown). Endothelial cells were detected using either CD34 or CD31 (Supplemental Fig. 1). The islet CD34+ vessels were larger than the vessels in the surrounding exocrine regions in control donors and were widely scattered throughout the islet (Fig. 1A–C; CD34). Islet vessel diameter, density, and area were examined by donor age and diabetes status with no significant differences observed by donor age in either group (Supplemental Fig. 2). Larger islet vessels were observed in control donors (Fig. 2A and B) compared with islet vessels in diabetic islets (Fig. 2C and D). Quantification of islet vessel diameters showed a significant difference with smaller vessels in islets from type 1 diabetes donors compared with control islets (6.2 ± 0.8 µm vs. 7.6 ± 1.1 µm, respectively; Fig. 2E). Vessel density was significantly higher in type 1 diabetes donors compared with control donors (9.3 ± 0.2 × 10–4 vessels/µm2 vs. 5.3 ± 0.6 ×104 vessels/µm2 islet area, respectively; Fig. 2F). The overall mean islet vessel area (%) was similar between control donors and donors with type 1 diabetes (8.8 ± 2.0% vs. 9.7 ± 1.7%, respectively; p=0.22; Fig. 2G).
Figure 2.
Islet vessels in control donors and donors with type 1 diabetes. Sections were stained and image analysis performed as described in section “Materials and Methods” for 16 control donors (N=10 islets/donor) and 17 donors with type 1 diabetes (N=7–20 islets/donor). Representative islets are shown from 2 control donors (A–B) and 2 diabetic donors (C–D). Islet vessel diameters from diabetic donors were significantly smaller than in control islets (E, ***p<0.001). Vessel density was significantly increased in diabetic islets compared with control islets (F, ***p<0.001). Islet vessel area (%) was not significantly different between donor groups (G). Scale bars = 100 µm. Abbreviations: SCG3, secretogranin 3; SMA, smooth muscle actin; INS, insulin; CD34, vascular endothelium.
Further studies were conducted to validate initial findings with increased islet sample sizes based on a previous study indicating an optimal sample size of 30 islets.31 Slides were reimaged and islets photographed and analyzed by a second observer (L.N.) using 5–35 islets per donor (n=3 control donors, n=4 donors with type 1 diabetes). Representative examples of vessel density are shown in Supplemental Fig. 3. Mean vessel density was similar from 5 to 35 islets examined from a single donor (Supplemental Fig. 3A). Mean islet vessel density and diameters were similar within donors examining 10 to 30 islets (Supplemental Fig. 3B and C).
Islet and Peri-islet Exocrine Vessel Parameters Were Different in Control Donors But Not in Donors With Type 1 Diabetes
Islet vessel diameters were significantly larger in islets compared with the peri-islet exocrine regions in control donors (7.6 ± 1.1 µm, 5.4 ± 0.2 µm, respectively; Fig. 3A). Islet vessel density was also significantly less than exocrine vessel density in control donors (5.3 ± 0.6 × 104 vessels/µm2, 9.4 ± 1 × 104 vessels/µm2, respectively; Fig. 3B). Significant differences were not observed between islet and exocrine vessel diameters for donors with type 1 diabetes (Fig. 3C and D) nor between controls and diabetic donor groups for exocrine vessel diameter, density, or area (Supplemental Fig. 4).
Figure 3.
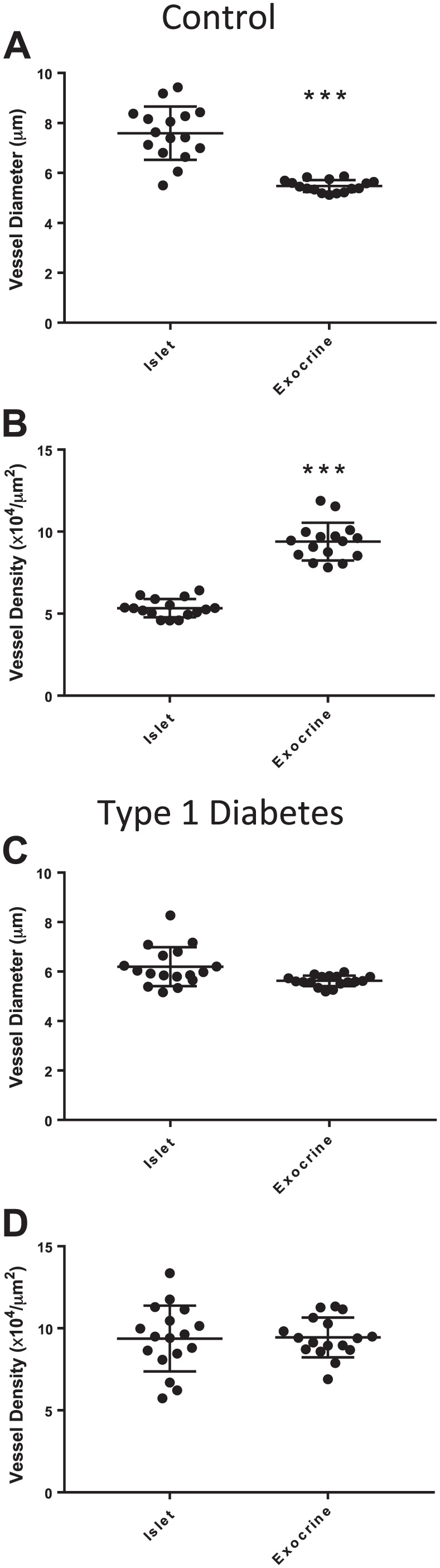
Islet and peri-islet exocrine vessels in control donors and donors with type 1 diabetes. Sections were stained and image analysis performed as described in section “Materials and Methods” for 16 control donors (N=10 islets/donor) and 17 donors with type 1 diabetes (N=7–20 islets/donor). Islet vessel diameters were significantly larger than peri-islet exocrine vessel diameters in control donors (A, ***p<0.001). Islet vessel density was significantly decreased compared with peri-islet exocrine vessel density in control islets (B, ***p<0.001). Islet and peri-islet exocrine vessel diameter and density were not significantly different in donors with type 1 diabetes (C–D, p>0.05). Scale bar = 100 µm.
Diabetes Duration and Vessel Parameters
The potential relationship between vessel morphology and type 1 diabetes duration was also examined. Islet vessel diameter tended to decrease with increasing diabetes duration, though the correlation was not significant (p=0.052; Fig. 4A). Islet vessel density was significantly increased with longer diabetes duration (p<0.05; Fig. 4B).
Figure 4.
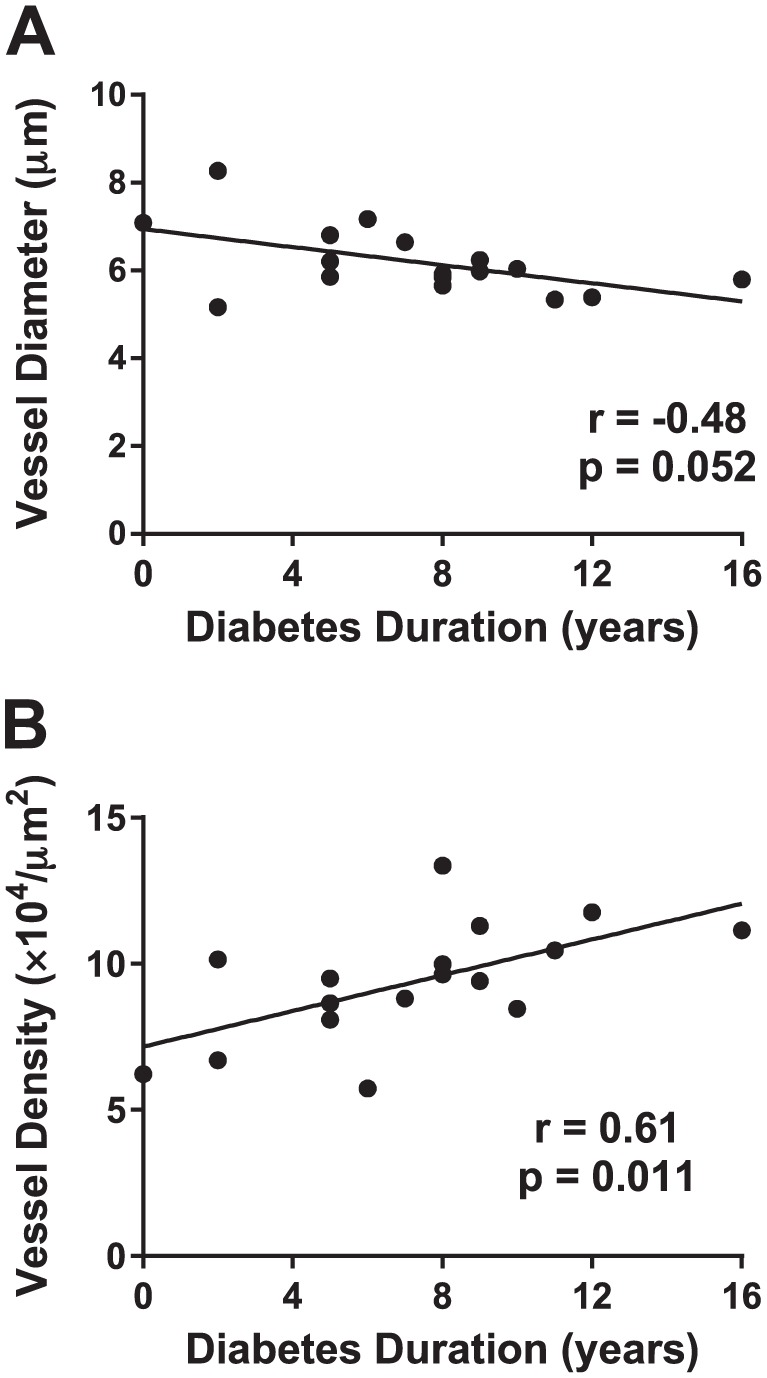
Diabetes duration and islet vessel diameter and density. Sections were stained and image analysis performed as described in section “Materials and Methods” for 17 donors with type 1 diabetes (N=7–20 islets/donor). Islet vessel diabetes (A) and density (B) were plotted as a function of diabetes duration (years), and correlation analysis was performed using Spearman’s test with linear regression lines shown.
Beta-cells in Islets From Donors With Type 1 Diabetes
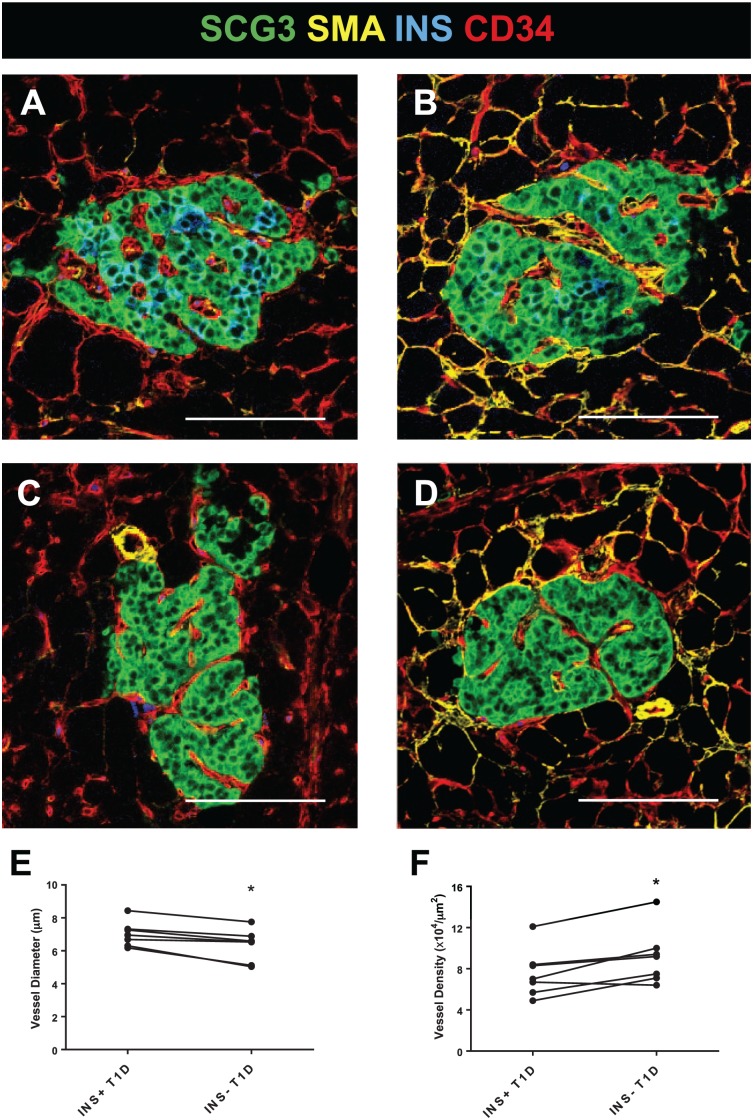
Seven (41%) of the 17 donors with type 1 diabetes had variable numbers of residual beta-cells (INS+) within islets (n=5–9 INS+ islets/donor; Supplemental Table 1; Fig. 5). INS+ islet vessel diameters were significantly larger (Fig. 5E) and had lower densities (Fig. 5F) compared with INS– islets within the same donor (n=5–6 INS– islets/donor). Significant correlations were not found between vessel diameter or vessel density versus islet insulin area (%; Supplemental Fig. 5). INS+ islets from type 1 diabetes donors were further subtyped by insulitis status (CD3+/–). INS+CD3+ islets were identified in 5 (71%) of the 7 diabetic donors (Supplemental Table 1). INS+CD3+ islets were infrequent (n=28 INS+CD3+ total from all 5 of the donors examined, n=4–7 islets/donor). The INS+CD3+ islet vessels were similar in diameter and density to those in INS+CD3– islets (Supplemental Fig. 6).
Figure 5.
Beta-cells maintain vessel morphology in diabetic islets. Pancreas sections were stained, and image analysis performed as described in section “Materials and Methods” for 7 donors with type 1 diabetes (N=5–15 INS+ islets/donor, N=4–6 INS– islets/donor; Supplemental Table 1). Representative INS+ (A–B) and INS– (C–D) islets are shown from 2 diabetic donors. Significant differences were observed for vessel diameter (E, *p<0.05) and density (F, *p<0.05) by INS phenotype. Scale bars = 100 µm. Abbreviations: SCG3, secretogranin 3; SMA, smooth muscle actin; INS, insulin; CD34, vascular endothelium.
Islet SMA Area Increased Significantly With Age
Islet SMA area (%) was variable between islets and was not significantly different between controls and donors with type 1 diabetes (Fig. 6A). Correlation analysis addressing an effect of age for the two donor groups did indicate a significant positive relationship for SMA area in control donors (r = 0.58, p=0.018) and donors with type 1 diabetes (r = 0.50, p=0.047; Fig. 3B).
Figure 6.
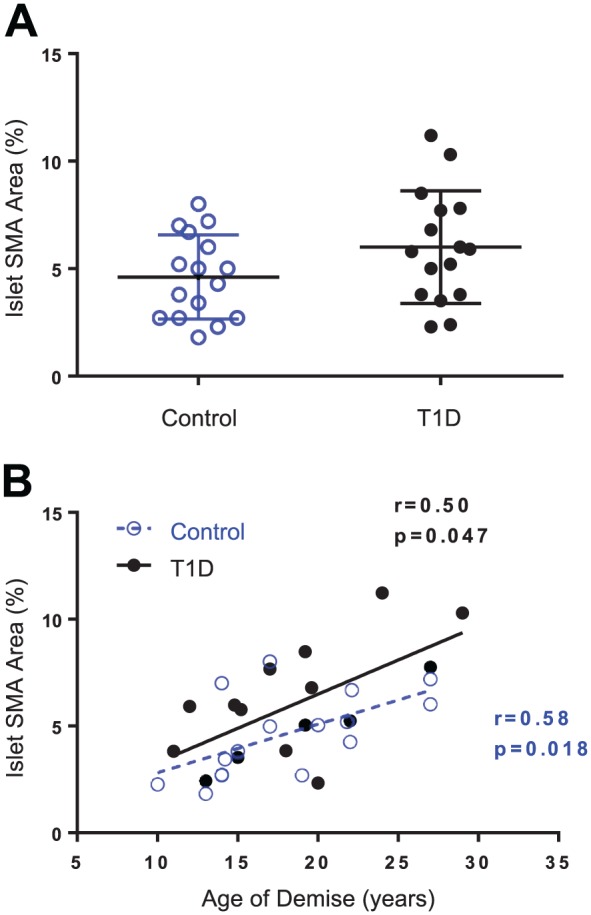
Islet SMA area and donor age. Pancreas sections were stained and image analysis performed as described in section “Materials and Methods” for 16 control donors (N=10 islets/donor) and 17 donors with type 1 diabetes (N=7–20 islets/donor). Islet SMA area (%) was plotted by donor group (A) and by age (years) for control donors (blue open circles, A) and diabetic donors (black closed circles, B). Islet SMA area was not significantly different between donor groups (Student’s t-test, p=.151) but was significantly correlated with donor age in both groups (B). Linear regression line plotted with Spearman’s r and p values, control slope = 0.23 ± 0.08, T1D slope = 0.32 ± 0.1. Abbreviations: SMA, smooth muscle actin; T1D, type 1 diabetes.
Discussion
The study reported herein examined the morphology of pancreatic islet and exocrine vessels in organ donors with and without type 1 diabetes. While exocrine vessel diameters and density were similar between control and diabetic donors, significant abnormalities of the islet microvasculature morphology were observed in the patients with type 1 diabetes, with vessels having smaller diameters and higher density. These changes were significantly decreased with the presence of residual beta-cells. These findings suggest residual islet beta-cells may have a critical role in maintenance of islet vessel morphology, or alternatively, changes to the islet microvasculature may contribute to beta-cell loss.
The islet vessel diameters for control donors reported in this study using formalin-fixed paraffin sections (7.6 ± 1.1 µm) were higher than those determined using fixed pancreas slices from patients undergoing partial pancreatectomy (5.1 ± 0.2 μm).10 Methodological differences in sample processing (paraffin 4 μm vs. agarose-embedded 120 μm vibratome sections), staining (CD34 vs. lectin staining), and image analysis procedures could potentially explain the differences in absolute islet vessel diameter. Interestingly, mean islet vessel areas were remarkably similar between these two studies (9.7 ± 1.7% vs. 9.1 ± 0.5%) despite a large difference in control donor ages (18.0 ± 5.0, N=16, vs. 62.8 ± 5.0, N=4; mean ± SD). These data support our finding that donor age did not significantly impact vessel parameters defined by CD34+ staining. Cohrs et al. also found that islet size did not impact islet vessel area in either mouse or human pancreas.10
The islet microvasculature in patients with type 2 diabetes has been studied by two groups, and one showed an increase in vessel density compared with control donors.20 Our results obtained from patients with type 1 diabetes also showed a significant increase in vessel density compared with control donors, suggesting a potential link between dysglycemia and vessel density. Due to the decrease in vessel diameter, islet vessel areas (%) were similar between control and type 1 diabetes islets. These data contrast with a recent study showing increased islet vessel area in donors with type 2 diabetes.23 Islets with increased vessel numbers and smaller diameters could imply increased vessel tortuosity following beta-cell loss. The physiological consequences of these morphological changes in islet vasculature remodeling with beta-cell loss are unknown but could impact functions of the remaining neuroendocrine cells, including alpha-cells.
The interdependence between beta-cells and vascular endothelial cells is well-described (reviewed in9,14). The development, maintenance, and proper function of the islet vasculature is dependent upon vascular endothelial growth factor (VEGF)-A signaling, which is largely produced by beta-cells.32 Insulin is also a known vasodilator via activation of endothelial nitric oxide (NO) synthase after insulin receptor binding.33 A major finding of our study showed that islets from diabetic donors with residual beta-cells had vessel diameters and densities more similar to control islets. Such findings suggest a normal islet microenvironment may be more conducive to beta-cell longevity.
Species differences in islet vessel SMA area are known with 3-fold greater SMA area in humans compared with rodents.21 While not different between donor groups, the SMA area did significantly increase with age in both control and diabetic donors. Sympathetic innervation of islet smooth muscle cells has been proposed to be the primary mechanism by which the sympathetic arm of the peripheral nervous system inhibits insulin secretion in humans, that is, vasoconstriction and reduced blood flow rate.21 In addition to the loss of vasodilatory mediators released by beta-cells, smaller vessels in islets of donors with type 1 diabetes could impact responses to autonomic or sensory neurotransmitters by alpha- and delta-cells (glucagon, somatostatin, respectively). Such findings could also have importance in islet transplantation as a negative correlation was observed between donor age and vascular density when human islets were transplanted under the mouse kidney capsule.34
In summation, several key alterations of the human islet microvasculature were identified in patients with type 1 diabetes. Residual beta-cells in islets from patients with type 1 diabetes were associated with islet vessels having greater diameters and decreased density comparable to islets from control donors. These findings confirm a critical relationship between beta-cells and maintenance of the islet microvasculature. Furthermore, the physiological significance of higher SMA area in older donors may underscore poorer islet transplantation survival from aged donors. Future studies are planned to examine potential mechanisms underlying these morphological findings including expression of VEGF or relevant VEGF receptors and neural input to the vascular smooth muscle cells in islets from patients with type 1 diabetes.
Supplementary Material
Supplemental Material, DS_10.1369_0022155418778546 for Islet Microvasculature Alterations With Loss of Beta-cells in Patients With Type 1 Diabetes by Joseph S. Canzano, Lith H. Nasif, Elizabeth A. Butterworth, Dongtao A. Fu, Mark A. Atkinson, and Martha Campbell-Thompson in Journal of Histochemistry & Cytochemistry
Acknowledgments
The authors acknowledge Dr. Stephanie Filipp for expert statistical assistance, Dr. Amanda Posgai for expert editorial assistance, Kamal Nasif for image analysis, and the organ donors and their families who made this research possible. This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by JDRF. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors have contributed to this article as follows: JSC performed the IHC, designed the image analysis, analyzed data, and wrote the manuscript; EAB and DAF performed IHC and reviewed the manuscript; LHN analyzed data and reviewed the manuscript; MAA reviewed the manuscript; MC-T designed the experiments, analyzed data, and wrote the manuscript; and all authors have read and approved the manuscript as submitted. MC-T is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of these data and the accuracy of the data analysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; UC4 DK 104155 01, 1OT2 TR001773 to MC-T) and Helmsley Charitable Trust (2015PG-T1D052 to MC-T).
ORCID iDs: MA Atkinson  https://orcid.org/0000-0001-8489-4782
https://orcid.org/0000-0001-8489-4782
M Campbell-Thompson  https://orcid.org/0000-0001-6878-1235
https://orcid.org/0000-0001-6878-1235
Contributor Information
Joseph S. Canzano, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida
Lith H. Nasif, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida
Elizabeth A. Butterworth, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida
Dongtao A. Fu, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida
Mark A. Atkinson, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida Department of Pediatrics, College of Medicine, University of Florida, Gainesville, Florida.
Martha Campbell-Thompson, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, Florida.
Literature Cited
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis-Richardson AG, Triplett EW. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia. 2015;58(7):1386–93. doi: 10.1007/s00125-015-3614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonifacio E, Mathieu C, Nepom GT, Ziegler AG, Anhalt H, Haller MJ, Harrison LC, Hebrok M, Kushner JA, Norris JM, Peakman M, Powers AC, Todd JA, Atkinson MA. Rebranding asymptomatic type 1 diabetes: the case for autoimmune beta cell disorder as a pathological and diagnostic entity. Diabetologia. 2017;60(1):35–8. doi: 10.1007/s00125-016-4144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdul-Rasoul M, Habib H, Al-Khouly M. “The honeymoon phase” in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7(2):101–7. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 6. Chmelova H, Cohrs CM, Chouinard JA, Petzold C, Kuhn M, Chen C, Roeder I, Kretschmer K, Speier S. Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes. 2015;64(6):2148–60. doi: 10.2337/db14-1055. [DOI] [PubMed] [Google Scholar]
- 7. Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Källskog Ö, Lau J, Liljebäck H, Palm F, Quach M, Sandberg M, Strömberg V, Ullsten S, Carlsson PO. Pancreatic islet blood flow and its measurement. Ups J Med Sci. 2016;121(2):81–95. doi: 10.3109/03009734.2016.1164769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes Metab. 2017;19(Suppl 1):124–36. doi: 10.1111/dom.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogan MF, Hull RL. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia. 2017;60(6):952–9. doi: 10.1007/s00125-017-4272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohrs CM, Chen C, Jahn SR, Stertmann J, Chmelova H, Weitz J, Bähr A, Klymiuk N, Steffen A, Ludwig B, Kamvissi V, Wolf E, Bornstein SR, Solimena M, Speier S. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017;158(5):1373–85. doi: 10.1210/en.2016-1184. [DOI] [PubMed] [Google Scholar]
- 11. Azizoglu DB, Chong DC, Villasenor A, Magenheim J, Barry DM, Lee S, Marty-Santos L, Fu S, Dor Y, Cleaver O. Vascular development in the vertebrate pancreas. Dev Biol. 2016;420(1):67–78. doi: 10.1016/j.ydbio.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes Obes Metab. 2008;10(Suppl 4):119–27. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 13. Tang SC, Baeyens L, Shen CN, Peng SJ, Chien HJ, Scheel DW, Chamberlain CE, German MS. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia. 2017;61(1):168–81. doi: 10.1007/s00125-017-4409-x. [DOI] [PubMed] [Google Scholar]
- 14. Zanone MM, Favaro E, Camussi G. From endothelial to beta cells: insights into pancreatic islet microendothelium. Curr Diabetes Rev. 2008;4(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15. Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31(4):705–14. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 16. Bonner-Weir S, Sullivan BA, Weir GC. Human islet morphology revisited: human and rodent islets are not so different after all. J Histochem Cytochem. 2015;63(8):604–12. doi: 10.1369/0022155415570969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31(10):883–9. [DOI] [PubMed] [Google Scholar]
- 18. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103(7):2334–9. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–10. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J Histochem Cytochem. 2015;63(8):637–45. doi: 10.1369/0022155415573324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14(1):45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7(1):e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah P, Lueschen N, Ardestani A, Oberholzer J, Olerud J, Carlsson PO, Maedler K. Angiopoetin-2 signals do not mediate the hypervascularization of islets in type 2 diabetes. PLoS ONE. 2016;11(9):e0161834. doi: 10.1371/journal.pone.0161834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, Crawford J, Schatz D, Pugliese A, Atkinson M. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28(7):608–17. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell-Thompson ML, Montgomery EL, Foss RM, Kolheffer KM, Phipps G, Schneider L, Atkinson MA. Collection protocol for human pancreas. J Vis Exp. 2012;(63):e4039. doi: 10.3791/4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasserfall C, Montgomery E, Yu L, Michels A, Gianani R, Pugliese A, Nierras C, Kaddis JS, Schatz DA, Bonifacio E, Atkinson MA. Validation of a rapid type 1 diabetes autoantibody screening assay for community based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol. 2016;185(1):33–41. doi: 10.1111/cei.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–31. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–80. [DOI] [PubMed] [Google Scholar]
- 30. Legland D, Beaugrand J. Automated clustering of lignocellulosic fibres based on morphometric features and using clustering of variables. Ind Crops Prod. 2013;45:253–61. doi: 10.1016/j.indcrop.2012.12.021. [DOI] [Google Scholar]
- 31. Meier DT, Entrup L, Templin AT, Hogan MF, Samarasekera T, Zraika S, Boyko EJ, Kahn SE. Determination of optimal sample size for quantification of β-cell area, amyloid area and β-cell apoptosis in isolated islets. J Histochem Cytochem. 2015;63(8):663–73. doi: 10.1369/0022155415585995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–85. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 33. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001;276(32):30392–8. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 34. Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87(12):5418–23. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DS_10.1369_0022155418778546 for Islet Microvasculature Alterations With Loss of Beta-cells in Patients With Type 1 Diabetes by Joseph S. Canzano, Lith H. Nasif, Elizabeth A. Butterworth, Dongtao A. Fu, Mark A. Atkinson, and Martha Campbell-Thompson in Journal of Histochemistry & Cytochemistry