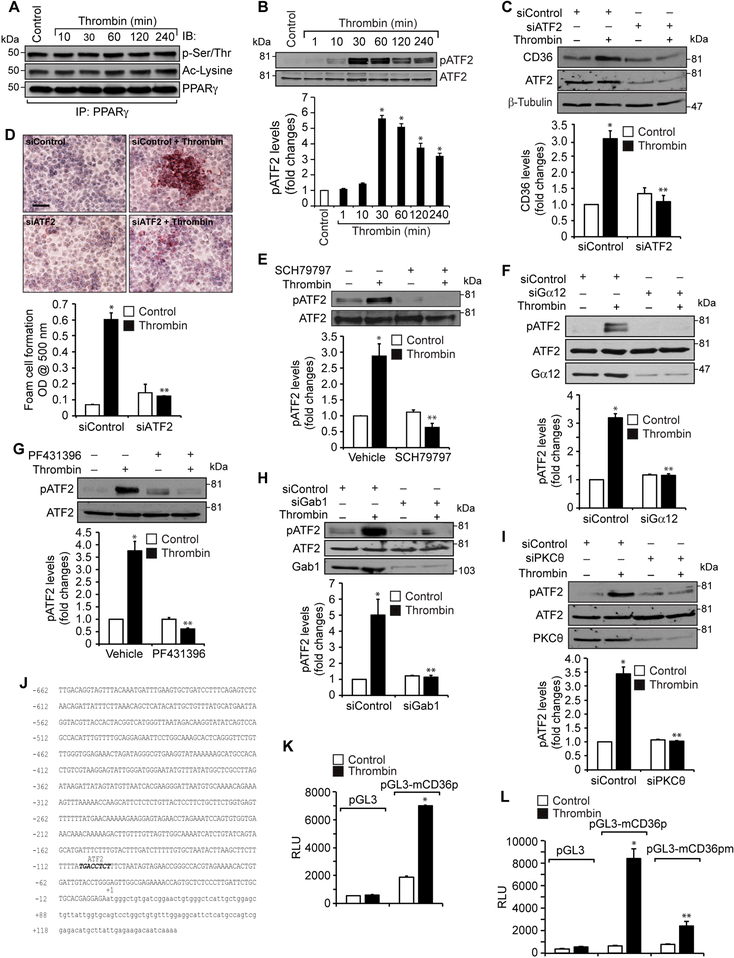
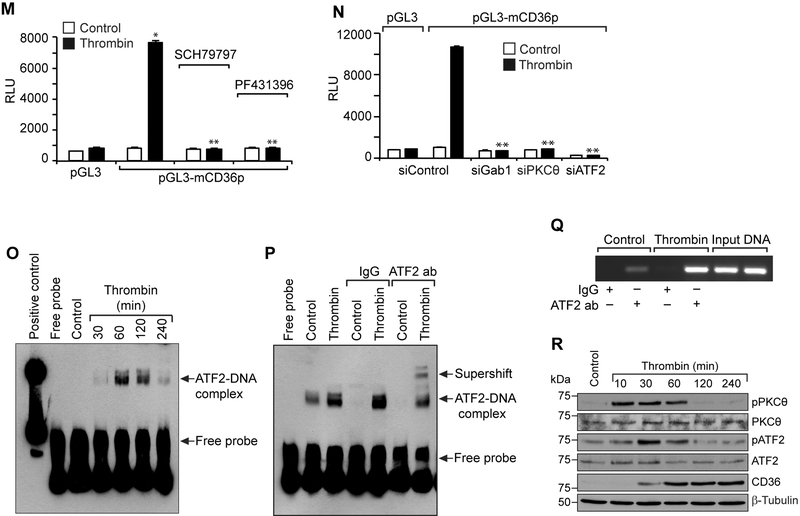
Figure 5. Par1, Gα12, Pyk2, Gab1, PKCα and ATF2 mediate thrombin-induced CD36 promoter activity.
A. Equal amounts of protein from control and the indicated time periods of thrombin-treated cells were immunoprecipitated with anti-PPARγ antibodies and the resulting immunocomplexes were analyzed by Western blotting for pSer/Thr or acetyl lysine antibodies and the blot was normalized to PPARγ levels. B. Equal amounts of protein from control and the indicated time periods of thrombin-treated cells were analyzed by Western blotting for pATF2 levels and the blot was normalized to its total levels. C & D. Cells were transfected with siControl or siATF2 (100 nM), quiesced, treated with and without thrombin for 1 hr or 4 hrs and analyzed for CD36 levels and foam cell formation. The CD36 blot was reprobed for ATF2 and β-tubulin levels to show the effect of the siRNA on its target and off target molecules levels. E & G. Quiescent cells were treated with and without thrombin in the presence and absence of SCH79797 (10 μM) or PF431396 (5 μM) for 1 hr and analyzed by Western blotting for pATF2 levels and normalized to its total levels. F, H & I. Cells were transfected with siControl, siGα12, siGab1 or siPKCθ (100 nM), quiesced, treated with and without thrombin and analyzed by Western blotting for pATF2 levels. The blots were reprobed for ATF2, Gα12, Gab1 or PKCθ to show the effects of the siRNAs on their target and off target molecules levels. J. CD36 promoter encompassing from -662 nt to +147 nt was cloned, sequenced and analyzed by TRANSFAC for transcriptional factors binding elements. K. The CD36 promoter encompassing from −662 nt to +147 nt was cloned into pGL3 vector and the RAW264.7 cells were transfected with the empty vector or pGL3-mCD36 promoter plasmids, growth-arrested, treated with and without thrombin for 6 hrs and the luciferase activity was measured. L. The RAW264.7 cells were transfected with empty vector, pGL3-mCD36 or pGL3-mCD36m (mutant for ATF2-binding site) plasmids, growth-arrested, treated with and without thrombin for 6 hrs and the luciferase activity was measured. M. All the conditions were the same as in panel K except that after transfection and quiescence, cells were treated with and without thrombin in the presence and absence of SCH79797 (10 μM), PF431396 (5 μM) for 6 hrs and the luciferase activity was measured. N. All the conditions were the same as in panel K except that after transfection with the plasmids, cells were again transfected with siGab1, siPKCθ or siATF2 (100 nM), quiesced, treated with and without thrombin for 6 hrs and analyzed for luciferase activity. O. Nuclear extracts of control and various time periods of thrombin treated cells were analyzed by EMSA for ATF2 binding using ATF2 binding site at −107 nt as a biotin labeled oligonucleotide probe. P. Nuclear extracts of control and thrombin-treated cells were analyzed for the presence of ATF2 in the protein-DNA complexes by supershift EMSA. Q. Control and thrombin-treated cells were analyzed for ATF2 binding to CD36 promoter by ChIP assay. R. Primary peritoneal macrophages from WT mice were treated with and without thrombin for the indicated time periods and analyzed by Western blotting for pPKCθ pATF2 or CD36 levels and normalized to their total levels or β-tubulin. The bar graphs represent Mean ± S.D. values of three experiments. *, p < 0.05 vs control or siControl or vector; **, p < 0.05 vs Thrombin, siControl + Thrombin or pGL3-mCD36p + Thrombin. Scale bar is 50 μm.