Abstract
Objectives
Iron deficiency (ID) is an emerging problem in patients with congestive heart failure (CHF) and can be a potential therapeutic target. As ID is highly prevalent in the society, it is hypothesized that Indian patients with CHF have high prevalence of ID.
Methods
CHF patients (n = 275) were selected and underwent laboratory evaluation including hemoglobin concentration, serum iron, transferrin, ferritin, B12 and folate level.
Results
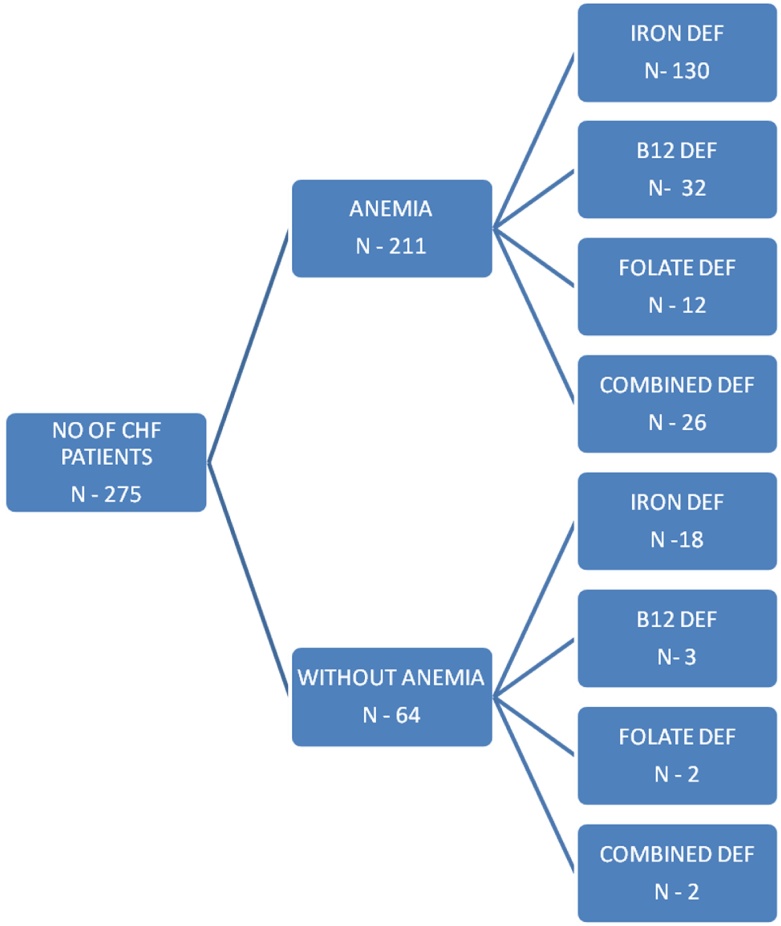
Two hundred and seventy-five patients with heart failure (mean age - 62.72, mean Hb- 10.54 g/dl, 188 males [68.36%] and 87 females [31.64%]) were enrolled in the study. 211 out of 275 (76.7%) were found to be anemic. Out of 275 patients. 148 (53.8%) were diagnosed with iron deficiency. 12.7% (n = 35) were B12 deficient and 5.1% (n = 14) were folate deficient. In the anemic group, ID was present in 130 patients (61.61%), B12 deficiency in 32 patients (15.16%) and folate deficiency in 12 patients (5.68%). In the group of patients without anemia, ID was present in 18 patients (28.12%) while B12 and folate deficiency was present in 3 (4.68%) and 2 (3.12%) patients, respectively.
Conclusion
Iron deficiency is present in substantial number while B12 and folate account for a few number of cases. Substantial number of patients without anemia were found to be iron deficient.
Keywords: Anemia, Iron, B 12, Folate, Heart failure
1. Background
Heart disease is increasing rapidly in prevalence. According to the American Heart Association, heart failure affects nearly 5.7 million Americans of all ages1, and is responsible for more hospitalizations than all forms of cancer combined. With improved survival of patients with acute myocardial infarction and with a population that continues to age, heart failure will be continuing to increaseas a major health problem.2, 3, 4, 5 In India, we do not have data regarding the exact prevalence and incidence of heart failure. With higher propensity for cardiovascular diseases and aging population the burden of congestive heart failure (CHF) is likely to be higher in comparison to the western population.6 As India is a population billionaire, the country accounts for the largest number of anemic persons in the world. The prevalence of anemia in children <5 years is 75%, women 15–49 years is 51%, pregnant women is 87% and maternal deaths from anemia/year is 22,000.7 Iron deficiency (ID) is an emerging problem in patients with heart failure and can be a potential therapeutic target. However, not much is known about the prevalence, predictors, and prognosis of ID in patients with congestive heart failure (CHF). ID is common in patients with heart failure, relates to disease severity, and is a strong and independent predictor of outcome.8 The prevalence and potential importance of ID per se, irrespective of hemoglobin, are currently a subject of interest in heart failure. However, data on this topic are scarce and only a few studies have reported on ID as a predictor of outcome in CHF. 9, 10, 11
There is a paucity of data available about the prevalence and characteristics of anemia in CHF patients in India. The present study was designed to know the prevalence of this important comorbidity and that of underlying nutritional deficiencies (serum iron, vitamin B12 and folate) in patients of CHF.
2. Material and methods
The study was done at Dharma Vira Heart Centre, Sir Ganga Ram Hospital, New Delhi. Patients coming to the institute either in OPD or in wards with CHF were subjected to thorough clinical evaluation and complete laboratory evaluation for anemia profile. It was an observational study, starting from 1st July 2015 to 30th June 2016.
All consecutive patients having clinical criteria for heart failure (according to the Framingham criteria), who were 18 years or older in age and were in NYHA functional class II- IV at the time of enrolment were included in this study. They were included regardless of the presence of anemia. Subjects with any of the following were excluded from the study- acute coronary syndrome with heart failure; myocardial infarction in the last three months; severe comorbidities like chronic kidney disease, chronic lung disease, chronic liver disease; severe uncontrolled heart failure with ejection fraction (EF) <30%; red blood cells (or other blood component) transfusions or erythropoietin therapy within 3 months prior to enrollment; iron, vitamin B12 and folate supplements used to treat anemia within 3 months; complex cyanotic congenital heart diseases; known malignant, hematological or other active neoplasia; and immunosuppressive therapy, chemotherapy or radiotherapy within 3 months prior to enrollment. Pregnant women were also excluded from the study.
Upon recruitment, all the patients underwent the following laboratory evaluation:
-
1
Hemoglobin concentration
-
2
Hematocrit
-
3
Mean corpuscular volume
-
4
Mean corpuscular hemoglobin concentration
-
5
Red cell distribution width
-
6
Serum iron concentration
-
7
Serum transferrin saturation
-
8
Serum ferritin saturation
-
9
Total iron binding capacity
-
10
Serum vitamin B12 level
-
11
Serum folate level
2.1. Definition of Anemia, iron, Vit B12 and folate deficiency
-
1
Hemoglobin <13 gm/dl (males) and < 12 gm/dl (females) - Anemia (WHO criteria)
-
2
Serum ferritin < 100 ng/ml- ID
-
3
Serum ferritin 100–300 ng/ml with transferrin saturation < 20%- ID
-
4
Serum Vit B12 < 200 pg/ml-vitamin B12 deficiency
-
5
Serum folate < 4 ng/ml – folate deficiency
2.2. Outcomes measured
A total number of 275 patients were taken in the study. The study population was categorized into two groups as either anemic or non-anemic. Each group was further sub categorized as having iron, B12, folate deficiency or combined iron and B12/folate deficiency according to the definitions.
2.2.1. Statistical methods
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. Data was presented as mean ± SD, frequencies and percentages.
3. Results
Table 1 describes the mean value and the standard deviation of the various study parameters. Prevalence of anemia, B12, folate and iron deficiency in the study population in aggregate is given in Table 2. Fig. 1 shows the number of patients of iron, B12 and folate deficiency in the anemic and non-anemic groups.
Table 1.
Clinical and laboratory characteristics of the study patients.
| Parameters | Values* |
|---|---|
| Age (years) | 62.7 ± 13.6 |
| Hemoglobin (gm/dL) | 10.5 ± 2.2 |
| Hematocrit (%) | 33.2 ± 6.4 |
| Mean corpuscular volume (fL) | 88.2 ± 65.7 |
| Mean corpuscular hemoglobin construction (g/dL) | 31.5 ± 1.3 |
| Red cell distribution width (%) | 17.0 ± 4.3 |
| Serum iron (mcg/dL) | 61.0 ± 36.0 |
| Serum transferrin (%) | 19.2 ± 10.6 |
| Serum ferritin (ng/mL) | 178.3 ± 177.1 |
| Serum total iron binding capacity (mcg/dL) | 381.4 ± 96.3 |
| Serum vitamin B12 (pg/mL) | 701.4 ± 442.5 |
| Serum folate (ng/mL) | 13.5 ± 5.1 |
all values are mean ± standard deviation.
Table 2.
Prevalence of anemia, iron, vitamin B12, folate deficiency and combined iron and B12/folate deficiency in the study population.
| Studied abnormality | Prevalence* (total N = 275) |
|---|---|
| Anemia | 211 (76.7) |
| Iron deficiency | 148 (53.8) |
| Folate deficiency | 14 (5.1) |
| Vitamin B12 deficiency | 35 (12.7) |
| Combined iron and vitamin B12 deficiency | 19 (6.9) |
| Combined iron and folate deficiency | 9 (3.2) |
| Combined Iron and vitamin B12 or folate deficiency | 28 (10.1) |
All values are actual numbers with percentages in parentheses.
Fig. 1.
Number of patients of CHF in anemic and non-anemic groups and each further subdivided according to iron, B12 and folate deficiency and combined iron and vitamin B12 or folate deficiency.
Overall, 76.7% patients had anemia. ID was found in 53.8% patients, 12.7% patients had vitamin B12 deficiency and 5.1% patients had folate deficiency.
Among the anemic subjects, ID was present in 61.6%, B12 deficiency in 15.2% and folate deficiency in 5.7%. In the non-anemic group, ID was present in 28.1% whereas B12 and folate deficiencies were present in 4.7% and 3.1% patients, respectively.
According to the Kuppuswamy score, the patient strata was found to be in upper for 49 patients (17.81%), upper middle for 118 patients (42.90%), lower middle for 69 patients (25.09%), upper lower for 33 patients (12%) and lower for 6 patients (2.18%).
Out of 275 patients, 147 (53.45%) were vegetarian and the remaining 128 (46.55%) were non-vegetarian. Non-vegetarian patients were at less risk for having B12 deficiency while vegetarians were at increased risk. No definite correlation could be found for type of diet and presence of anemia, iron deficiency and folate deficiency.
4. Discussion
Anemia is commonly present in CHF patients. ID in CHF is associated with reduced iron stores in the bone marrow and the heart. ID is an independent risk factor for severity and worsening of the CHF. Currently used intravenous (IV) iron preparations are safe and effective in treating the ID in CHF whereas less is known on the effectiveness of oral iron. In CHF IV iron correction of ID is associated with improvement in functional status, exercise capacity, quality of life. 12
The European Society of Cardiology Guidelines for heart failure 2012 recommended a diagnostic work-up for ID in patients with suspected heart failure. Iron absorption from oral iron preparations is generally poor, and up to 60% of patients can have gastrointestinal side effects. Evidence for clinical benefits using oral iron is lacking. IV iron sucrose has consistently been shown to improve exercise capacity, cardiac function, symptom severity, and quality of life. Similar findings were observed recently for IV ferric carboxymaltose (FCM) in patients with systolic heart failure and impaired LVEF in the double-blinded, placebo-controlled FAIR-HF and CONFIRM-HF trials. IV iron is preferable although confirmation in longer clinical trials is awaited. 13
FCM is a dextran-free iron-carbohydrate complex (which has a very low risk for hypersensitivity reactions) with a small proportion of the reported adverse effects in a large number of subjects who received FCM and thus it may be considered a safe drug. 14
ID occurs when the dietary intake is inadequate, during times of digestive blood loss or menstrual periods, and during states that excessively increased iron requirements, particularly during childhood growth or pregnancy. In patients with chronic diseases, on the other hand, iron may become immobilizable as a consequence of chronic inflammation thus leading to a functional ID. 15
About half of all patients with CHF have either absolute ID or functional ID defined as ferritin <100 μg/L or transferrin saturation <20% and serum ferritin 100–300 μg/L, respectively. This finding is only partly associated with the presence of anemia. Indeed, many heart failure patients present with ID, many with anemia, and some of these with both. The absence of iron in the blood of patients with heart failure may also be reflected as reduced iron load in the bone marrow and in the myocardium. At the level of the bone marrow, erythropoietin controls synthesis of new erythrocytes 15.
Erythropoietin is synthesized predominantly in the peritubular endothelium cells of the kidney and during anemia, the healthy kidney can increase erythropoietin production by at least 10-fold.16
Serum levels of hepcidin, a regulatory protein of iron metabolism synthesized by the liver, do not differ between anemic and non-anemic subjects, and there is no relationship between serum hepcidin and the change in either hemoglobin or serum C-reactive protein levels during the progression of HF. Hepcidin is an acute phase reactant whose secretion increases with systemic inflammation.17
Our study was conducted in 275 patients of CHF with their thorough evaluation of anemia and serum iron, vitamin B12 and folate deficiency along with emphasis on dietary and socioeconomic history. Previous studies have found prevalence of anemia to vary considerably from 4%–55% owing to different selection criterion.18 Our study found 76.7% prevalence of anemia as defined by the WHO criterion (Hb <13 gm/dL in males and < 12 gm/dL in females). ID was found in 53.8% patients in our study. The prevalence of ID was similar to as described by McDonagh et al 13, but it was lower than that describe in a recent study by Sharma et al 19 This study had included 150 CHF patients and ID was present in 76% of the patients (Table 3).
Table 3.
Comparison between the prevalence of ID in CHF patients between our study and the study done by Sharma et al.
| Study characteristics | Our study | Sharma et al 19 |
|---|---|---|
| Number of patients | 275 | 150 |
| With Iron deficiency | 148 (53.8%) | 114 (76%) |
| Iron deficiency in anemic | 130 (61.6%) | 77 (51.3%) |
| Iron deficiency in non-anemics | 18 (28.12%) | 37 (24.7%) |
In comparison to the study by Sharma et al, we also measured the prevalence of vitamin B12 and folate deficiency in our patients. Overall, 12.7% patients had vitamin B12 deficiency (defined as serum vitamin B12 < 200 pg/ml) and 5.1% patients had folate deficiency (i.e. serum folate <4 ng/ml). A study done by Van der Waal et al20 in 610 heart failure patients had found the prevalence of B12, folate and ID to be 5%, 4% and 58%, respectively; these values were similar to those observed in our study, except for vitamin B12 deficiency which was more common in our patients.
Our study thus emphasizes the prevalence of anemia in majority (76.6%) of the CHF patients. ID was found to be the most important cause in the anemic group (61.61%) while B12 and folate were causative in fewer patients. Importantly, 28.1% patients without anemia also had ID. This group is often the ignored one. Iron supplementation in iron deficient patients in both anemic and without anemia patients should be sought for.
5. Conclusion
Heart failure is a major cause of morbidity and mortality. Anemia is an important comorbidity. ID is common in patients with heart failure. While it is more prevalent in the anemic population, it is present in a substantial number of patients who do not have anemia. Targeting the ID can help ameliorate the adverse effects of heart failure
Source of support
Nil
Conflicts of interest
None. We confirm that the manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M. For the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow G.C. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb S.S., Khatta M., Friedmann E. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–1549. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 4.He J., Ogden L.G., Bazzano L.A., Vupputuri S., Loria C., Whelton P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 5.Masoudi F.A., Havranek E.P., Smith G. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S., Bahl A., Talwar K.K. Congestive heart failure in Indians: how do we improve diagnosis & management? Indian J Med Res. 2010;132:549–560. [PMC free article] [PubMed] [Google Scholar]
- 7.India’s Undernourished Children: A Call For Reform and Action, World Bank Report: http://siteresources.worldbank.org/healthnutritionandpopulation/Resource/2816271095698140167/IndiaUndernourishedChildrenFinal.pdf.
- 8.Klip I.T., Comin-Colet J., Voors A.A. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska E.A., Rozentryt P., Witkowska A. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;15:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 10.Okonko D.O., Mandal A.K., Missouris C.G., Poole-Wilson P.A. Disordered iron homeostatis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Parikh A., Natarajan S., Lipsitz S.R., Katz S.D. Iron deficiency in community dwelling U.S. Adults with self-reported heart failure in NHANES III: prevalence and association with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverberg D.S., Wexler D., Schwartz D. Is Correction of iron deficiency a New addition to the treatment of the heart failure? Int J Mol Sci. 2015;16:14056–14074. doi: 10.3390/ijms160614056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonagh T., Macdougall I.C. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. 2015;17:248–262. doi: 10.1002/ejhf.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toblli J.E., Angerosa M. Optimizing iron delivery in the management of anemia: patient considerations and the role of ferric carboxymaltose. Drug Des Devel Ther. 2014;8:2475–2491. doi: 10.2147/DDDT.S55499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebner N., von Haehling S. Iron deficiency in heart failure: a practical guide. Nutrients. 2013;5:3730–3739. doi: 10.3390/nu5093730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handelman G.J., Levin N.W. Iron and anemia in human biology: a review of mechanisms. Heart Fail Rev. 2008;13:393–404. doi: 10.1007/s10741-008-9086-x. [DOI] [PubMed] [Google Scholar]
- 17.Kroot J.J.C., Tjalsma H., Fleming R.E., Swinkels D.W. Hepcidin in human iron disorders: diagnostic implications. Clin Chem. 2011;57:1650–1669. doi: 10.1373/clinchem.2009.140053. [DOI] [PubMed] [Google Scholar]
- 18.Mishra T.K., Mishra S.K., Mohanty N.K., Rath P.K. Prevalence, prognostic importance and therapeutic implications of anemia in heart failure. Indian Heart J. 2005;57:670–674. [PubMed] [Google Scholar]
- 19.Sharma S.K., Agarwal S.K., Bhargava K., Sharma M., Chopra K., Arumugam G. Prevalence and spectrum of iron deficiency in heart failure patients in south Rajasthan. Indian Heart J. 2016;68:493–497. doi: 10.1016/j.ihj.2015.10.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Wal, Comin-Colet J., Klip I.T. Vitamin B12 and folate deficiency in chronic heart failure. Heart. 2015;101:302–310. doi: 10.1136/heartjnl-2014-306022. [DOI] [PubMed] [Google Scholar]