Fig 2. ETPs inhibit protein degradation in vitro and in cells.
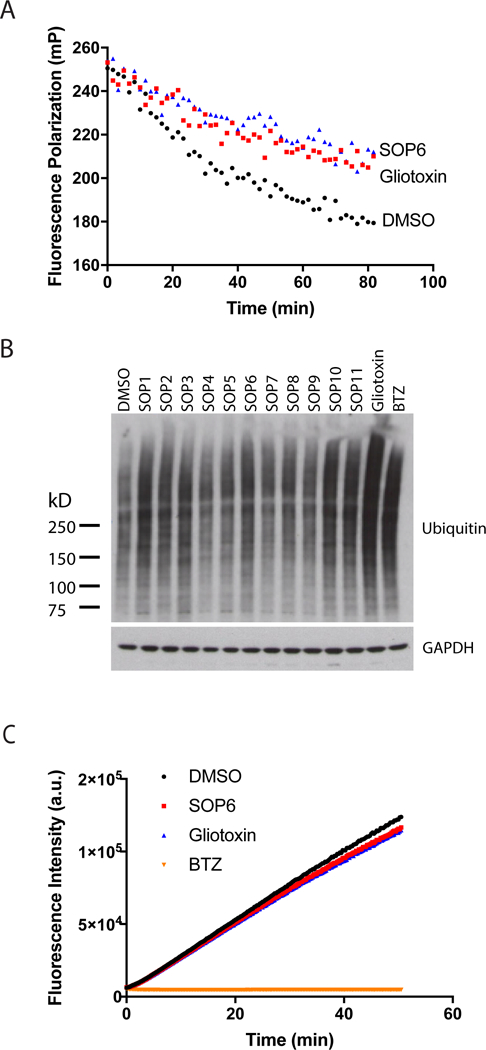
(A) SOP6 and gliotoxin block the processing of K48UbnGST–Wbp2 by the proteasome. K48UbnGST–Wbp2 was incubated with 26S proteasome at 37 °C in the absence or presence of SOP6 or gliotoxin (10 µM each). (B) ETP treatment caused accumulation of high-molecular weight ubiquitin conjugates. HCT116 cells were treated for 3 hours with 10 μM of the indicated ETPs, and the cell lysates were fractionated by SDS–PAGE and immunoblotted with antibodies against ubiquitin. (C) SOP6 and gliotoxin do not inhibit chymotrypsin-like activity of the proteasome. Suc-LLVY-amc (20 μM) was incubated with 15 nM purified human 26S proteasome in the absence or presence of SOP6, gliotoxin or BTZ (20 µM each).
