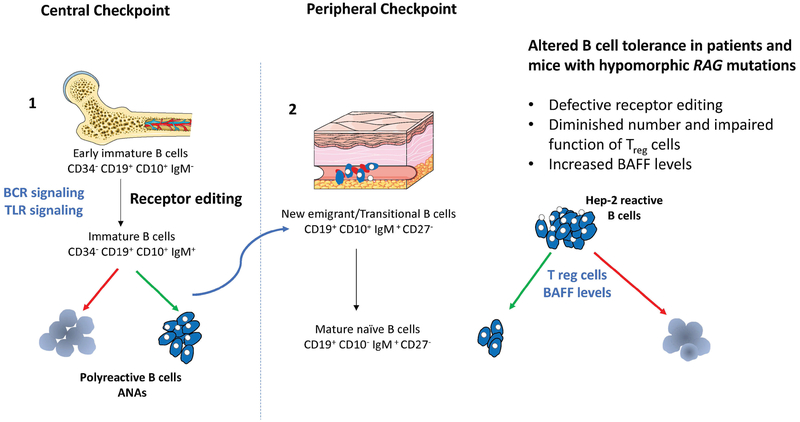
Figure 2. Schematic representation of B cell tolerance checkpoints.
Left panel: The majority of newly generated B cells in bone marrow express self-reactive antibodies including polyreactive specificities. At the first checkpoint, counterselection of highly polyreactive and self-reactive antibodies positive B cells relies on three mechanisms: 1) receptor editing, which involves RAG re-expression and depends on BCR cross-linking by self-antigens; 2) deletion of cells recognizing with high affinity self-antigens; and, 3) anergy, by which B cells become unresponsive to antigen. BCR synergizes with TLR signaling for the selection of autoreactive clones.
Right panel: Despite these mechanisms, some self-reactive immature B cells are released from the bone marrow and reach the periphery where they undergo a second checkpoint before maturing into naïve B cells. The number of self-reactive B cells, dramatically drops during this transition., as shown by the reduction in the proportion of B cells expressing immunoglobulins that recognize Hep-2 cells. Both Treg cells and levels of BAFF are involved in the maintenance of peripheral tolerance. In particular, high levels of BAFF are present in B cell lymphopenic hosts, and promote the survival of transitional B cells, including those expressing self-reactive specificities. Hypomorphic RAG mutations may interfere with both central and peripheral B cell tolerance as indicated on the right.