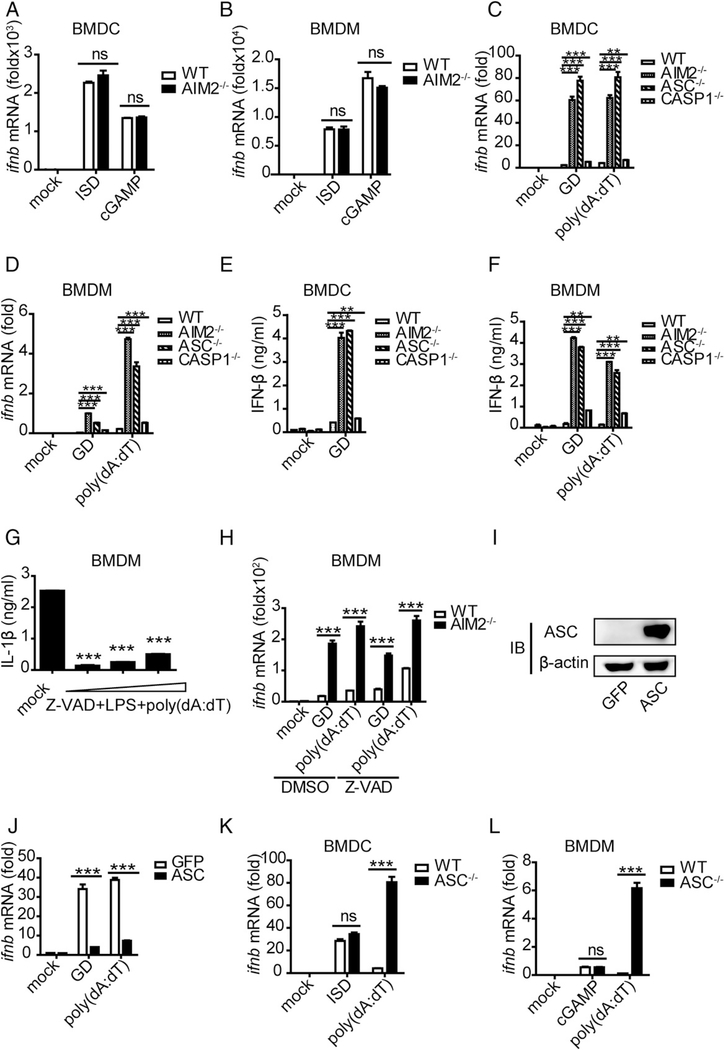
FIGURE 3.
The activated form of ASC performs an inhibitory role on IFN-β upon DNA ligand stimulation. (A and B) BMDCs and BMDMs isolated from WT and AIM2−/− mice were stimulated with ISD (1.5 μg/ml) and cGAMP (4 μg/ml), and IFN-β was measured by quantitative real-time PCR (qRT-PCR) 8 h after transfection. (C and D) BMDCs and BMDMs isolated from WT, AIM2−/−, ASC−/−, and Caspase-1−/− mice were stimulated with GD (1.5 μg/ml) and poly(dA:dT) (1.5 μg/ml), and IFN-β was measured by qRT-PCR 10 h after transfection. (E and F) BMDCs and BMDMs isolated from WT, AIM2−/−, ASC−/−, and Caspase-1−/− mice were stimulated with GD (1.5 μg/ml) and poly(dA:dT) (1.5 μg/ml), and IFN-β was measured by ELISA 10 h after transfection. (G) BMDMs isolated from WT mice were stimulated with different concentrations of a caspase inhibitor (Z-VAD-FAM) for 1 h and then stimulated with LPS for 2 h before being stimulated with poly(dA:dT) (1.5 μg/ml); IL-1β was measured by ELISA 6 h after transfection. (H) BMDMs isolated from WT and AIM2−/− mice were stimulated with DMSO or a caspase inhibitor (Z-VAD-FAM) for 1 h and then stimulated with GD (1.5 μg/ml) and poly(dA:dT) (1.5 μg/ml). IFN-β was measured by qRT-PCR 8 h after transfection. (I) Immunoblot of ASC and β-actin in RAW-GFP and RAW-ASC cell lysates. (J) RAW-GFP and RAW-ASC cells were stimulated with GD (1.5 μg/ml) and poly(dA:dT) (1.5 μg/ml), and IFN-β was measured by qRT-PCR 8 h after transfection. (K and L) BMDCs and BMDMs isolated from WT and ASC−/− mice were stimulated with ISD (1.5 μg/ml), cGAMP (4 μg/ml), and poly(dA:dT) (1.5 μg/ml), and IFN-β was measured by qRT-PCR 8 h after transfection. Data are representative of at least two independent experiments. The results are shown as mean ± SEM. **p < 0.01, ***p < 0.001, Student t test. ns, not significant.