Abstract
Although current guidelines advocate using the CHA2DS2-VASc score to assess the risk of stroke in patients with atrial fibrillation (AF), compared with transesophageal echocardiography (TEE), its ability to predict left atrial appendage thrombus (LAAT) is limited. We studied 3,324 consecutive patients with sustained AF from our prospective registry of patients who underwent first-time TEE-guided electrical cardioversion (ECV) from May 2000 through March 2012. The association of CHA2DS2-VASc score or TEE risk factors with the occurrence of LAAT was analyzed. The mean (SD) age was 69 (12.5) years and 67% were men. LAAT was identified in 49 (1.5%) during pre-ECV TEE. Compared with patients without LAAT, those with LAAT had lower peak left atrial appendage emptying velocity (LAAEV) (17.2 ± 8.5 vs 36.6 ± 20.8; p <0.001) and left ventricular ejection fraction (LVEF) (39.9 ± 17.6 vs 51.4 ± 13.7; p <0.001); their CHA2DS2-VASc score also was higher, but the difference was not statistically significant (3.6 ± 1.4 vs 3.2 ± 1.6; p = 0.06). Multivariate logistic regression analysis identified an LVEF ≤40% (adjusted odds ratio 2.48, 95% confidence interval 1.38 to 4.46), LAAEV 20.3 to 33.9 cm/s (odds ratio 12.19, 95% confidence interval 1.53 to 96.86), and LAAEV ≤20.2 cm/s as independent predictors of LAAT. An LAAEV cut-point of 20 cm/s and an LVEF ≤40% were optimal for detecting LAAT (sensitivity 75% and 62%; specificity 77% and 75%; area under the curve 0.822 and 0.776, respectively). On follow-up, LAAT was an independent risk factor of subsequent ischemic stroke but did not influence survival. In conclusion, reduced LVEF and reduced LAAEV are important pathophysiologic correlates of left atrial appendage thrombogenesis and subsequent ischemic stroke in patients who underwent TEE-guided ECV for AF.
Atrial fibrillation (AF) is a major public health problem in the developed world,1 and is associated with an increased risk of stroke,2 generally attributed to the formation of left atrial appendage thrombus (LAAT) and distal embolization.3 Current guidelines advocate using the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65 to 74 years, gender category) score for estimation of stroke risk in patients with AF.4 However, the discriminatory ability of the CHA2DS2-VASc score for predicting LAAT is modest with a c-statistic of 0.607.5 The role of transesophageal echocardiography (TEE) for the assessment of LAAT is well-established.6 We have demonstrated an independent association between TEE-measured left atrial appendage (LAA) emptying velocity (LAAEV) and subsequent ischemic stroke after successful electrical cardioversion (ECV) of AF.7 In this investigation, we sought to determine the ability of TEE risk factors, principally correlates of systolic (left ventricular ejection fraction [LVEF]) and diastolic (LAAEV) dysfunction,8 compared with the CHA2DS2-VASc score to predict LAAT before ECV for AF.
Methods
The study protocol was approved by the Mayo Clinic Institutional Review Board. We prospectively collected data from consecutive eligible patients with sustained AF who underwent TEE to exclude LAAT before restoration of sinus rhythm by ECV from May 2000 through March 2012. Sustained AF was defined as AF that fails to self-terminate. LAAT was defined echocardiographically as a well-circumscribed, often mobile, highly reflective mass of uniform consistency, with texture different from the atrial wall and with a border distinct from the surrounding structures in multiple imaging planes.9 We identified 3,340 consecutive patients with sustained AF who underwent first-time TEE-guided ECV during the study period. We excluded patients with history of complex congenital heart disease or LAA closure (n = 16), yielding a total of 3,324 patients.
The precardioversion TEE protocol has been described previously.7,10,11 Briefly, all patients underwent TEE immediately before ECV. Echocardiographic data, including LVEF and LAAEV, were routinely assessed. The peak LAAEV profiles were measured over 5 consecutive cardiac cycles using pulsed-wave Doppler interrogation with the sample volume positioned 1 cm within the orifice of the LAA. In the absence of an intracardiac thrombus, ECV was performed using established monophasic (2000) or biphasic (2001 to 2012) waveform protocols of the cardioversion unit for cardioversion of AF.12
The primary outcome of the study was LAAT, which was determined by TEE immediately before ECV. The secondary outcomes were first documented ischemic stroke and all-cause mortality. Stroke was defined as the acute onset focal neurologic deficits persisting more than 24 hours, and based on results of CT or MRI or autopsy (if available). Vital status was ascertained through 2014 by using the National Death Index.
Categorical variables were expressed as counts and percentages and compared by chi-square analysis or Fisher’s exact test, depending on the distribution of the data. Continuous variables were expressed as means (SD) or median and interquartile ranges. Variables were compared by using the Student’s t test or the Wilcoxon rank-sum test. Predictors of LAAT were assessed with a multivariable logistic regression analysis. The measurement of predictive accuracy of the echocardiographic parameters (e.g., LAAEV, LVEF) in screening for LAAT was assessed by using a receiver operating characteristic curve. Cox regression models were used to identify potential factors associated with ischemic stroke or with all-cause mortality. Results of these analyses were expressed as hazard ratios and 95% confidence intervals. Multicollinearity was examined by using a correlation matrix and diagnostic statistics. Survival-free estimates of neurologic events and death were computed by using the Kaplan-Meier method and compared with a log-rank test. A 2-tailed p value of <.05 was considered significant. All statistical analyses were performed by using the SAS version 9.4M3 (SAS Institute Inc., Cary, North Carolina) and R v3.1 (R Development Core Team, 2016, Vienna, Austria).
Results
Baseline characteristics and demographics of the study patients are listed in Table 1. The mean (SD) age was 69 (12.5) years and 67% were men. Overall, 71% of patients were receiving oral anticoagulation (OAC) therapy with warfarin or a novel oral anticoagulant at the time of TEE. LAAT was identified in 49 patients (1.5%) during precardioversion TEE. Compared with patients without LAAT, those with LAAT had lower mean (SD) LAAEV (17.2 [8.5] vs 36.3 [20.8] cm/s; p <0.001) and LVEF (39.9% [17.6%] vs 51.4% [13.7%]; p <0.001) and a higher nonsignificant mean (SD) CHA2DS2-VASc score (3.6 [1.4] vs 3.2 [1.6]; p = 0.06).
Table 1.
Baseline characteristics
| Characteristic | No LAA Thrombus | LAA Thrombus | P Value |
|---|---|---|---|
| (n = 3,275) | (n = 49) | ||
| Age (years) | 69.0 ± 12.5 | 67.8 ± 14.4 | .50 |
| Male sex | 2,183 (66.7%) | 36 (73.5%) | .36 |
| Body mass index (kg/m2) | 30.6 ± 6.8 | 31.3 ± 8.5 | .57 |
| Hypertension | 2,187 (66.8%) | 41 (83.7%) | .01 |
| Diabetes mellitus | 654 (20.0%) | 10 (20.4%) | .61 |
| Prior myocardial infarction | 528(16.1%) | 8 (16.3%) | .59 |
| Cardiomyopathy (ischemic, idiopathic) | 767 (23.4%) | 25 (51.0%) | <.001 |
| Heart failure | 1,408 (43.0%) | 35 (71.4%) | <.001 |
| Prior stroke | 385 (11.8%) | 6 (12.2%) | .82 |
| Smoker (current or former) | 1,566 (47.8%) | 23 (46.9%) | .51 |
| Chronic lung disease | 445 (13.6%) | 7 (14.3%) | .83 |
| Obstructive sleep apnea | 576 (17.6%) | 10 (20.4%) | .57 |
| Peripheral arterial disease | 360(11.0%) | 7 (14.3%) | .49 |
| Prior coronary bypass | 475 (14.5%) | 13 (26.5%) | .03 |
| CHA2DS2-VASc score | 3.2 ± 1.6 | 3.6 ± 1.4 | .06 |
| CHA2DS2-VASc score | .26 | ||
| 0 | 188 (5.7%) | 0 (0.0%) | |
| 1 | 284 (8.7%) | 4 (8.2%) | |
| 2 | 617 (18.8%) | 7 (14.3%) | |
| >2 | 2,186(66.8%) | 38 (77.6%) | |
| Precardioversion International Normalized Ratio | 1.91 ± 0.89 | 1.80 ± 0.81 | .46 |
| Duration of AF episode | .24 | ||
| >48 hours* | 410 (12.5%) | 3 (6.1%) | |
| >2days to <7 days | 806 (24.6%) | 9 (18.4%) | |
| >7 days to <1 year | 1,470 (44.9%) | 28(57.1%) | |
| >1 year | 103 (3.1%) | 1 (2.0%) | |
| Preprocedure medications | |||
| β-Blocker | 1,977 (60.4%) | 31 (63.3%) | .66 |
| Calcium-channel blocker (nondihydropyridine) | 1,080 (33.0%) | 17 (34.7%) | .88 |
| Statin | 1,087 (33.2%) | 14 (28.6%) | .54 |
| Angiotensin converting enzyme inhibitor or Angiotensin II receptor blocker | 1,361 (41.6%) | 32 (65.3%) | .001 |
| Antiplatelet | 1,792 (54.7%) | 29 (59.2%) | .57 |
| Warfarin | .29 | ||
| No Warfarin* | 990 (30.2%) | 11 (22.4%) | |
| Warfarin started in hospital | 1,279(39.1%) | 21 (42.9%) | |
| Warfarin with subtherapeutic INR | 727 (22.2%) | 15 (30.6%) | |
| Warfarin with therapeutic INR | 275 (8.4%) | 2(4.1%) | |
| Novel oral anticoagulant | 32 (1.0%) | 0 (0.0%) | |
| Antiarrhythmic | 1,052(32.1%) | 11 (22.5%) | .17 |
| Hemodynamics | |||
| Heart rate (beats/min) | 92.7 (22.7) | 87.2 (18.5) | .38 |
| Echocardiography | 36.6 ± 20.8 | 17.2 ± 8.5 | <.001 |
| Left atrial appendage emptying velocity (cm/s) | |||
| Left atrial appendage emptying velocity | <.001 | ||
| Tertile 1, ≤20.8 (cm/s) | 821 (25%) | 39 (79.6%) | |
| Tertile 2, 20.8–46.4 (cm/s) | 1,638 (50.0%) | 9 (18.4%) | |
| Tertile 3, >46.4 (cm/s) | 821 (25.1%) | 1 (2.0%) | |
| Spontaneous echo contrast (LA or LAA) | 1,423 (43.5% | 38 (77.6%) | <.001 |
| Left ventricular ejection fraction (%) | 51.4 ± 13.7 | 39.9 ± 17.6 | <.001 |
| Left ventricular ejection fraction ≤40% | 745 (22.7%) | 24 (49.0%) | <.001 |
| Left atrial enlargement (moderate or higher) | 2040 (70.00%) | 41 (93.2%) | <.001 |
| Mitral regurgitation (moderate or higher) | 844 (25.8%) | 19 (38.8%) | .05 |
| INR at discharge | 1.95 ± 0.85 | 1.94 ± 0.71 | .52 |
| Discharge medications | |||
| β-Blocker | 1,937(59.1%) | 33 (67.3%) | .24 |
| Calcium-channel blocker (nondihydropyridine) | 608 (18.6%) | 11 (22.4%) | .47 |
| Statin | 1,183 (36.1%) | 13 (26.5%) | .18 |
| Angiotensin converting enzyme inhibitor or Angiotensin II receptor blocker | 1,554 (47.5%) | 30 (61.2%) | .06 |
| Antiplatelet | 1,707 (52.1%) | 30(61.2%) | .25 |
| Low-molecular-weight heparin | 1,076 (32.9%) | 24 (49.0%) | .03 |
| Warfarin | 2,861 (87.4%) | 47 (95.9%) | .12 |
| Novel oral anticoagulant | 39 (1.2%) | 0 (0.0%) | |
| Antiarrhythmic | 1,143 (34.9%) | 12 (24.5%) | .17 |
Values are mean (SD), median (IQR) or n (%).
Hypertension was defined in accordance with 2016 ACC/AHA guidelines.
Reference category.
We performed a univariate logistic regression analysis to identify associations between precardioversion clinical and echocardiographic features with LAAT (Table 2). Candidate variables (characteristics with a p value <0.10) were entered into stepwise multivariable logistic regression models. This analysis identified moderately reduced peak LAAEV (20.3 to 33.9 cm/s) (odds ratio [OR] 12.19, 95% confidence interval [CI] 1.53 to 96.86), severely reduced LAAEV ≤20.2 cm/s (OR 65.85, 95% CI 8.93 to 485.39), and moderately reduced or lower LVEF (defined as ejection fraction ≤40%) (OR 2.48, 95% CI 1.38 to 4.46) as independent predictors of LAAT (c-statistic, 0.849) (Table 3A). When CHA2DS2-VASc components were included in lieu of CHA2DS2-VASc score, results were similar; LVEF ≤40% or CHF and reduced LAAEV remained significant predictors of LAAT (Table 3B).
Table 2.
Univariate analysis for the prediction of LAA thrombus, ischemic stroke, and death
| Characteristic | LAA Thrombus | Ischemic Stroke | Death | |||
|---|---|---|---|---|---|---|
| (n = 49) | P Value | (n = 182) | P Value | (n = 1,118) | P Value | |
| Odds ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Demographics | ||||||
| Age | 1.00 (0.98–1.02) | .80 | 1.04 (1.02–1.05) | <.001 | 1.07 (1.06–1.07) | <.001 |
| Male sex | 1.32 (0.78–2.24) | .30 | 0.79 (0.59–1.07) | .13 | 0.83 (0.73–0.93) | .002 |
| Body mass index | 1.00 (0.95–1.04) | .86 | 1.00 (0.98–1.02) | .99 | 0.97 (0.96–0.98) | <.001 |
| Hypertension | 2.19(1.17–4.09) | .01 | 1.46 (1.05–2.03) | .02 | 1.32(1.16–1.50) | <.001 |
| Diabetes mellitus | 0.87 (0.48–1.60) | .66 | 0.98 (0.67–1.43) | .90 | 1.58 (1.38–1.80) | <.001 |
| Prior myocardial infarction | 1.56 (0.89–2.75) | .12 | 1.47(1.02–2.11) | .04 | 1.79 (1.56–2.06) | <.001 |
| Cardiomyopathy (ischemic, idiopathic) | 3.41 (1.93–6.00) | <.001 | 0.77(0.53–1.11) | .17 | 1.15 (1.01–1.32) | .04 |
| Heart failure | 3.43 (2.00–5.87) | <.001 | 1.22 (0.91–1.63) | .19 | 1.61 (1.43–1.81) | <.001 |
| Prior stroke | 1.05 (0.52–2.13) | .88 | 2.10(1.46–3.02) | <.001 | ||
| Smoker | 1.08 (0.74–1.56) | .70 | 1.12(0.84–1.50) | .43 | 1.15 (1.02–1.29) | .02 |
| Chronic lung disease | 1.28 (0.70–2.35) | .42 | 1.14(0.72–1.80) | .58 | 1.80(1.54–2.11) | <.001 |
| Obstructive sleep apnea | 0.89 (0.50–1.61) | .71 | 1.28 (0.88–1.85) | .20 | 0.88 (0.74–1.04) | .13 |
| Peripheral arterial disease | 1.35 (0.60–3.03) | .47 | 1.39(0.91–2.14) | .13 | 1.77(1.51–2.08) | <.001 |
| Prior coronary bypass | 2.71 (1.62–4.52) | <.001 | 1.34 (0.93–1.99) | .12 | 2.01 (1.75–2.31) | <.001 |
| CHA2DS2-VASc score | 1.17(1.01–1.36) | .04 | 1.24(1.13–1.36) | <.001 | 1.38 (1.33–1.43) | <.001 |
| 0* | ||||||
| 1* | 2.13 (0.59–7.74) | .25 | 2.03 (1.16–3.56) | .01 | ||
| 2 | 0.81 (0.23–2.77) | .73 | 3.17(0.97–0.37) | .06 | 2.57(1.53–4.31) | <.001 |
| >2 | 1.23 (0.44–3.48) | .69 | 4.46 (1.42–14.01) | .01 | 5.44 (3.32–8.92) | <.001 |
| Duration of atrial fibrillation episode | ||||||
| >48 h (vs <48 h) | 1.62 (0.88–2.96) | .12 | 0.96 (0.83–1.09) | .51 | ||
| <48 h* | ||||||
| >2 days to <7 days | 0.90 (0.56–1.44) | .66 | 1.08 (0.89–1.32) | .43 | ||
| >7 days to <1 year | 0.80 (0.52–1.23) | .30 | 0.91 (0.75–1.09) | .31 | ||
| >1 year | 0.46(0.14–1.50) | .20 | 0.81 (0.54–1.22) | .31 | ||
| Preprocedure medications | ||||||
| β-Blocker | 1.52 (0.91–2.54) | .11 | 1.40 (1.03–1.90) | .03 | 0.92 (0.82–1.04) | .17 |
| Calcium-channel blocker (nondihydropyridine) | 1.16(0.71–1.89) | .56 | 1.48(1.10–1.99) | .01 | 0.99(0.87–1.12) | .89 |
| Statin | 0.85 (0.51–1.41) | .53 | 0.95 (0.69–1.32) | .76 | 0.93 (0.81–1.06) | .28 |
| Angiotensin converting enzyme inhibitor or Angiotensin II receptor blocker | 2.53 (1.54–4.15) | <.001 | 1.12(0.83–1.50) | .46 | 1.21 (1.07–1.36) | .002 |
| Antiplatelet | 1.60 (0.97–2.62) | .06 | 1.01 (0.75–1.35) | .95 | 1.22 (1.09–1.38) | .001 |
| Warfarin | 1.11 (0.48–2.57) | .81 | 0.92 (0.86–0.98) | .006 | ||
| Antiarrhythmic | 0.66(0.39–1.12) | .12 | 0.75 (0.54–1.05) | .09 | 0.94 (0.83–1.07) | .37 |
| Hemodynamics | ||||||
| Heart rate−1 | 1.01 (0.99–1.03) | .32 | 1.00 (0.99–1.01) | .90 | 0.997 (0.994–0.999) | .01 |
| Echocardiography | ||||||
| Left atrial appendage emptying velocity−1 | 1.12(1.09–1.15) | <.001 | 1.01 (1.01–1.02) | .003 | 1.01 (1.01–1.02) | <.001 |
| Tertile 1, ≤20.8 | 78.21 (10.72–570.33) | <.001 | 1.56(1.11–2.19) | .009 | ||
| Tertile 2, 20.8–46.4 | 13.55 (1.72–107.15) | .01 | 1.42 (0.97–2.07) | .07 | ||
| Tertile 3, >46.4* | ||||||
| Spontaneous echo contrast (LA or LAA) | 5.17 (2.70–9.88) | <.001 | 1.40 (1.01–1.95) | .004 | 1.24 (1.08–1.42) | .002 |
| Left atrial thrombus | 3.28 (1.77–6.07) | <.001 | 6.89 (0.96–49.19) | .05 | 2.50 (0.63–10.02) | .20 |
| Left atrial appendage thrombus | 2.74 (1.29–5.83) | .009 | 1.06 (0.66–1.71) | .80 | ||
| Ejection fraction−1 | 1.05 (1.03–1.06) | <.001 | 1.00 (0.99–1.01) | .67 | 1.01 (1.01–1.02) | .002 |
| Ejection fraction ≤40% | 3.37 (1.89–6.01) | <.001 | 0.85 (0.59–1.22) | .37 | 1.26(1.10–1.44) | .001 |
| Mitral regurgitation (≥moderate) | 1.27 (0.76–2.12) | .37 | 1.15 (0.84–1.58) | .38 | 1.42 (1.26–1.60) | <.001 |
| International Normalized Ratio at discharge | 1.08 (0.81–1.45) | .60 | 1.03 (0.85–1.23) | .80 | 1.09(1.01–1.17) | .03 |
CI = confidence interval.
Reference category.
Table 3.
(A) Multivariate regression analysis for the prediction of left atrial appendage thrombus (stepwise logistic), ischemic stroke, and death (Cox) (model 1). (B) Multivariate regression analysis for the prediction of left atrial appendage thrombus (stepwise logistic), ischemic stroke, and death (Cox) (model 2)
| A | ||||||
| Variables | Left atrial appendage thrombus | Ischemic Stroke | Death | |||
| (n = 49) | P Value | (n = 182) | P Value | (n = 1,118) | P Value | |
| Odds ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| CHA2DS2-VASc score | ||||||
| 0* | ||||||
| 1*,† | 2.30 (0.63–8.35) | .21 | 2.03 (1.16–3.57) | .01 | ||
| 2 | 0.56(0.18–1.63) | .50 | 3.19 (0.97–10.49) | .06 | 2.32 (1.38–3.92) | .002 |
| >2 | 0.65 (0.19–2.30) | .29 | 4.35 (1.38–13.76) | .01 | 4.96(3.02–8.15) | <.001 |
| Left atrial appendage emptying velocity | ||||||
| Tertile 3, >34* | ||||||
| Tertile 2, 20.3–33.9 | 12.19(1.53–96.86) | .02 | 1.21 (0.81–1.81) | .36 | 1.21 (1.04–1.39) | .02 |
| Tertile 1, ≤20.2 | 65.85 (8.93–485.39) | <.001 | 1.46 (1.03–2.07) | .03 | 1.39(1.19–1.62) | <.001 |
| Ejection fraction ≤40% | 2.48 (1.38–4.46) | <.001 | 0.72 (0.50–1.05) | .09 | 1.09 (0.95–1.25) | .23 |
| Left atrial appendage thrombus | 2.84 (1.30–6.19) | .009 | 0.86(0.51–1.39) | .51 | ||
| C-statistic | 0.849 | 0.726 | 0.859 | |||
| B | ||||||
| Variables | Left atrial appendage thrombus | Ischemic Stroke | Death | |||
| (n = 49) | P Value | (n = 182) | P Value | (n = 1,118) | P Value | |
| Odds ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Congestive heart failure or Ejection fraction ≤40% | 1.78 (1.28–3.53) | .03 | 1.10(0.82–1.47) | .55 | 1.52(1.36–1.69) | <.001 |
| Hypertension | 1.65 (1.07–5.41) | .04 | 1.32 (0.93–1.87) | .12 | .98(0.87–1.12) | .79 |
| Age > = 75 years | 2.06 (1.40–3.03) | <.001 | 3.76 (3.20–4.43) | <.001 | ||
| Diabetes | 1.19(0.83–1.69) | .35 | 1.39 (1.22–1.58) | <.001 | ||
| Prior Stroke | 1.83 (1.28–2.62) | .001 | 1.24 (1.07–1.43) | .005 | ||
| Vascular disease | 1.15 (0.85–1.56) | .36 | 1.52(1.35–1.71) | <.001 | ||
| Age 65–74 y | 1.21 (0.80–1.82) | .37 | 1.83 (1.55–2.18) | <.001 | ||
| Sex (female) | 1.17(0.86–1.59) | .33 | 1.04(0.92–1.17) | .57 | ||
| Left atrial appendage emptying velocity | ||||||
| Tertile 3, >34* | ||||||
| Tertile 2, 20.3–33.9 | 1.24 (0.88–1.73) | .22 | 1.10(0.96–1.24) | .18 | ||
| Tertile 1, ≤20.2 | 56.81 (7.66–421.57) | <.001 | 1.32 (1.02–1.87) | .03 | 1.19(1.03–1.36) | .02 |
| Left atrial appendage thrombus | 3.21 (1.48–6.95) | .003 | 1.10(0.67–1.78) | .71 | ||
| C-statistic | 0.844 | 0.704 | 0.842 | |||
CI = confidence interval.
Reference category.
CHA2DS2-VASc score reference value for LAA thrombus (0–1).
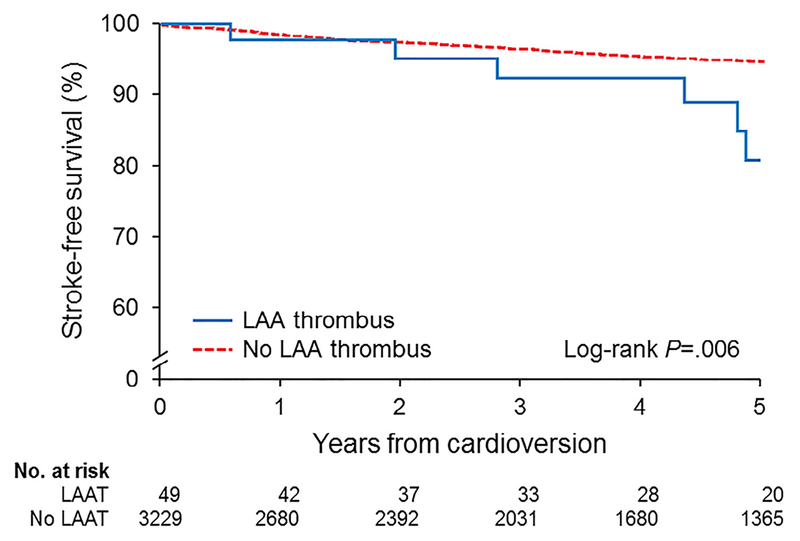
During a mean (SD) follow-up of 4.8 (3.6) years, 182 ischemic strokes occurred. The cumulative stroke-free survival in the entire population is shown in Figure 1. By Kaplan-Meier analysis, the stroke-free survival rate was significantly lower for patients with LAAT compared with those without LAAT (log-rank test, p = 0.006). Results of univariate Cox regression analysis are listed in Table 2. In multivariable Cox regression analysis, LAAT (hazard ratio [HR] 2.84, 95% CI 1.30 to 6.19), CHA2DS2-VASc score >2 (HR 4.35, 95% CI 1.38 to 13.76]), and severely reduced LAAEV ≤20.2 cm/s (HR 1.46, 95% CI 1.03 to 2.07) emerged as independent predictors of ischemic stroke (c-statistic, 0.726).
Figure 1.
Kaplan-Meier analysis showing freedom from ischemic stroke in patients with or without LAAT. The survival curves show significantly lower freedom from stroke in the group with LAAT compared with the group without LAAT.
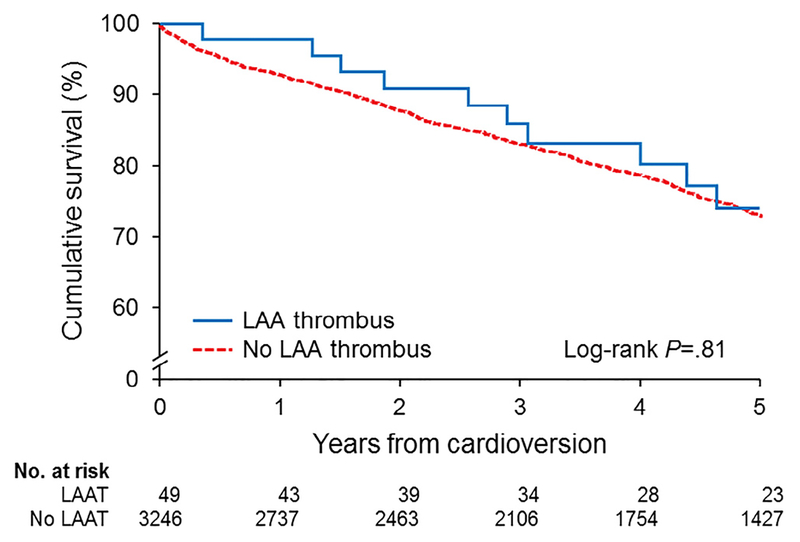
Of the 3,324 patients in the study, 1,118 (33.6%) died during follow-up. The cumulative survival of the entire cohort is illustrated in Figure 2. By Kaplan-Meier analysis, cumulative survival rates were not significantly different between patients with and without LAAT (p = 0.81). Multivariable Cox proportional hazards regression analysis (Table 3) showed that a CHA2DS2-VASc score of 1 (HR 2.03, 95% CI 1.16 to 3.57), CHA2DS2-VASc score of 2 (HR 2.32, 95% CI 1.38 to 3.92), CHA2DS2-VASc score >2 (HR 4.96, 95% CI 3.02 to 8.15), LAAEV ≤20.3 to 33.9 cm/s (HR 1.21, 95% CI 1.04 to 1.39), and LAAEV ≤20.2 cm/s (HR 1.39, 95% CI 1.19 to 1.62) were independent predictors of all-cause mortality (c-statistic, 0.859).
Figure 2.
Kaplan-Meier survival curves of patients with or without LAAT during precardioversion TEE. The survival curves show no significant difference in survival in the group with LAAT versus the group without LAAT.
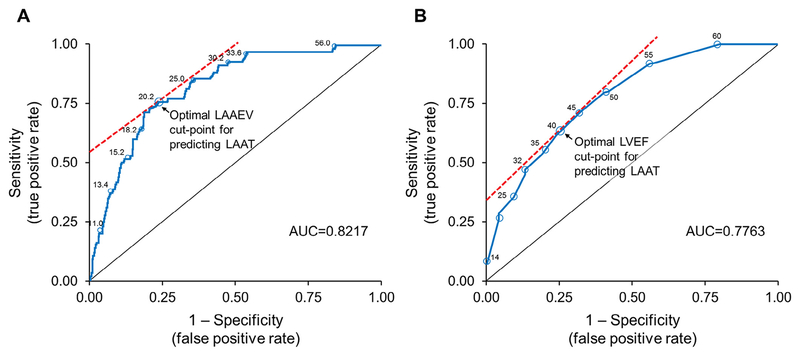
Receiver operating characteristic curve analysis showed good discriminatory capacity of LAAEV and fair for LVEF in predicting LAAT in patients with AF who underwent TEE-guided ECV. An LAAEV cut-point of 20.2 cm/s was optimal for detecting LAAT (sensitivity, 75%; specificity, 77%; area under the curve [AUC], 0.822) (Figure 3). An LVEF cut-point of 40% was also useful for detecting LAAT (sensitivity, 62%; specificity, 75%; AUC, 0.776) (Figure 3).
Figure 3.
(A) ROC curve for LAAEV to predict the presence of LAAT in patients with atrial fibrillation who underwent TEE-guided ECV. An LAAEV cut-point of 20.2 cm/s had 75% sensitivity and 77% specificity for predicting LAAT; AUC = 0.822. (B) ROC curve for LVEF. An LVEF cut-point of 40% had 62% sensitivity and 75% specificity for predicting LAAT; AUC = 0.776. ROC = receiver operating characteristic.
Discussion
In this large cohort of patients who underwent TEE-guided ECV for AF, the prevalence of LAAT among patients who underwent TEE was 1.5%. LAAT was strongly associated with reduced LVEF and reduced peak LAAEV, independent of other clinical and echocardiographic risk factors. A LAAEV cut-point of 20.2 cm/s and a LVEF cut-point of 40% provided optimal sensitivity and specificity for detecting LAAT. Reduced LAAEV, LAAT, and CHA2DS2-VASc score >2 were independent predictors of subsequent stroke. These results provide additional evidence that LVEF and LAAEV are not just pathophysiologic echocardiographic correlates of systolic and diastolic dysfunction8; rather, they may be considered surrogate markers of LAAT and subsequent ischemic stroke in patients who underwent TEE-guided ECV for AF.
Although LAAT is recognized as a major cause of stroke and thromboembolism in patients with AF,13 its true prevalence remains unknown. Previous studies have shown wide variation in diagnostic yield of TEE for LAAT in patients with AF, ranging from 0.5% to 15%.14–17 A recent meta-analysis that examined 20,516 patients with AF who underwent TEE from 72 studies reported a prevalence of LAAT of 9.8%.18 In contrast, Puwanant et al17 reviewed more than 1,000 preablation TEEs and reported that LAAT was present in 0.6% of patients with AF. Although the low prevalence of LAAT (1.5%) in our study was consistent with other studies of patients with AF who underwent preprocedural TEE,16,17 it contrasts with previous observations of patients with AF who underwent TEE generally for the detection of LAAT.6,19 This difference in prevalence rates of LAAT could be due to heterogeneity among the studies, level of oral OAC, and technological improvement in echocardiography over time. Although TEE screening for LAAT before cardioversion is recommended for patients with AF lasting ≥48 hours (or of unknown duration) who have not received OAC for at least 3 weeks,4 definitive evidence-based data for this recommendation are lacking. Previous studies have demonstrated that thrombus formation in the LAA has a dominant role in the thromboembolic risk associated with AF.3,20 Consistent with previous reports,13,19 in our study, lower LVEF and LAAEV were independently associated with LAAT formation. Similar to previous reports, most patients with LAAT had a CHA2DS2-VASc score >2. However, CHA2DS2-VASc score was not predictive of LAAT in the present study. Although patients with LAAT more frequently had hypertension, congestive heart failure, vascular disease, and lower LVEF, the mean CHA2DS2-VASc score was not significantly different between the 2 groups, consistent with previous observations that individual risk factors in the CHA2DS2-VASc score do not carry an equal risk.21
The most catastrophic complication of AF is embolic stroke.22 The detection of LAAT by TEE has important clinical and prognostic implications. Previous studies have established that LAAT is associated with increased risk of thromboembolic events.13,23 In the present study, we showed that LAAT, LAAEV, and CHA2DS2-VASc score were independently associated with increased risk of ischemic stroke. Despite the lack of association with LAAT, CHA2DS2-VASc score was a strong predictor of ischemic stroke. This suggests that the association of AF with thromboembolic risk is multifactorial and likely involves risk factors beyond the presence of LAAT and stasis of flow in the LAA.
Patients with AF have a higher mortality rate than those with sinus rhythm.24 The impact of LAAT on survival has not been specifically assessed previously. Theoretically, stasis of blood flow in the LAA may be a major determinant of excess mortality, potentially attributable to the risk of fatal stroke in patients with AF and greater burden of cardiovascular disease in patient with AF.25 However, in the present study survival was not significantly different between patients with LAAT versus without LAAT, likely because patients with LAAT are typically monitored more closely and receive more adequate OAC and for a longer period than those without LAAT, which could potentially minimize the risk of death from fatal stroke or subsequent embolization.
The pathogenesis of LAAT formation is a complex and incompletely understood phenomenon. Previous studies have shown that LAA flow velocity is markedly attenuated by elevated left ventricular filling pressure, resulting in LAA stasis, thereby increasing the risk of LAA thrombosis.26,27 Such a thrombogenic milieu may explain the pathophysiologic mechanism and predisposition for stroke, ostensibly due to thrombus formation in the LAA.28
Our findings support broader use of OAC in patients with reduced LVEF and static flow in the LAA, irrespective of CHA2DS2-VASc score or duration of AF. As LAAEV is a physiologic measurement of left atrial and diastolic function, we speculate that noninvasive measurements of left atrial and diastolic function (e.g., mitral inflow e-wave, e-wave deceleration time, mitral annular velocity, and left atrial dimension) could potentially serve similar function—that is, as adjunct to established stroke risk assessment tools to guide therapy in AF based on individual risk profiles.
Although our results are based on prospectively collected data, a number of potential limitations should be considered in the interpretation of these findings. Ours was a single-cohort study, which could have site-specific bias. Despite our relatively large sample size, the number of LAAT events was somewhat limited. Our results should therefore be considered hypothesis generating and require confirmation. The TEE procedures were performed during a 12-year period; thus, operator experience and technological advancements may have confounded results. Data regarding the duration or pattern of periprocedural OAC were not available, making it difficult to discern the impact of OAC on LAAT formation. However, the number of patients receiving OAC was similar in both groups, suggesting that other factors, such as AF recurrence and severity of atrial stasis over time, may have had a predominant role. AF duration could not be ascertained accurately from the medical records as many as 50% of cases of AF can be silent,29 and may explain the lack of association of AF duration with stroke in our study. Although TEE is the most sensitive and reliable technique to detect LAAT as a potential cardiac source of thromboembolism before ECV, it is a semi-invasive procedure with associated risks.
In conclusion, LAAT in patients who underwent TEE-guided cardioversion is uncommon. The present study showed that reduced LVEF and reduced LAAEV are important pathophysiologic correlates of LAA thrombogenesis and subsequent ischemic stroke in patients who underwent TEE-guided ECV for AF. These findings support broader use of OAC in these patients, irrespective of CHA2DS2-VASc score or duration of AF.
Acknowledgments
Dr. Melduni is supported by the National Institutes of Health (NIH) K01 HL 135288.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 5.Willens HJ, Gomez-Marin O, Nelson K, DeNicco A, Moscucci M. Correlation of CHADS2 and CHA2DS2-VASc scores with transesophageal echocardiography risk factors for thromboembolism in a multiethnic United States population with nonvalvular atrial fibrillation. J Am Soc Echocardiogr 2013;26:175–184. [DOI] [PubMed] [Google Scholar]
- 6.Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, Davidoff R, Erbel R, Halperin JL, Orsinelli DA, Porter TR, Stoddard MF. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–1420. [DOI] [PubMed] [Google Scholar]
- 7.Melduni RM, Lee HC, Bailey KR, Miller FA Jr, Hodge DO, Seward JB, Gersh BJ, Ammash NM. Real-time physiologic biomarker for prediction of atrial fibrillation recurrence, stroke, and mortality after electrical cardioversion: a prospective observational study. Am Heart J 2015;170:914–922. [DOI] [PubMed] [Google Scholar]
- 8.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284–1289. [DOI] [PubMed] [Google Scholar]
- 9.Seward JB, Khandheria BK, Oh JK, Freeman WK, Tajik AJ. Critical appraisal of transesophageal echocardiography: limitations, pitfalls, and complications. J Am Soc Echocardiogr 1992;5:288–305. [DOI] [PubMed] [Google Scholar]
- 10.Melduni RM, Chandrasekaran K, Friedman PA, White RD, Malouf JF, Hodge DO, Seward JB, Oh JK, Ammash NM. Does left atrial appendage peak emptying flow velocity predict the electrical energy required to achieve successful direct-current cardioversion in patients with sustained atrial fibrillation? J Am Soc Echocardiogr 2007;20:1004–1008. [DOI] [PubMed] [Google Scholar]
- 11.Melduni RM, Malouf JF, Chandrasekaran K, Bruce CJ, White RD, Law KK, Al Atawi FO, Somers VK, Gersh BJ, Hodge DO, Friedman PA, Seward JB, Ammash NM. New insights into the predictors of left atrial stunning after successful direct-current cardioversion of atrial fibrillation and flutter. J Am Soc Echocardiogr 2008;21:848–854. [DOI] [PubMed] [Google Scholar]
- 12.Gurevitz OT, Ammash NM, Malouf JF, Chandrasekaran K, Rosales AG, Ballman KV, Hammill SC, White RD, Gersh BJ, Friedman PA. Comparative efficacy of monophasic and biphasic waveforms for transthoracic cardioversion of atrial fibrillation and atrial flutter. Am Heart J 2005;149:316–321. [DOI] [PubMed] [Google Scholar]
- 13.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol 1998;31:1622–1626. [DOI] [PubMed] [Google Scholar]
- 14.Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol 1995;25:1354–1361. [DOI] [PubMed] [Google Scholar]
- 15.Rader VJ, Khumri TM, Idupulapati M, Stoner CN, Magalski A, Main ML. Clinical predictors of left atrial thrombus and spontaneous echocardiographic contrast in patients with atrial fibrillation. J Am Soc Echocardiogr 2007;20:1181–1185. [DOI] [PubMed] [Google Scholar]
- 16.Khan MN, Usmani A, Noor S, Elayi S, Ching CK, Di Biase L, Patel D, Burkhardt JD, Cummings J, Schweikert R, Saliba W, Natale A. Low incidence of left atrial or left atrial appendage thrombus in patients with paroxysmal atrial fibrillation and normal EF who present for pulmonary vein antrum isolation procedure. J Cardiovasc Electrophysiol 2008;19:356–358. [DOI] [PubMed] [Google Scholar]
- 17.Puwanant S, Varr BC, Shrestha K, Hussain SK, Tang WH, Gabriel RS, Wazni OM, Bhargava M, Saliba WI, Thomas JD, Lindsay BD, Klein AL. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol 2009;54:2032–2039. [DOI] [PubMed] [Google Scholar]
- 18.Di Minno MN, Ambrosino P, Dello Russo A, Casella M, Tremoli E, Tondo C. Prevalence of left atrial thrombus in patients with nonvalvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost 2016;115:663–677. [DOI] [PubMed] [Google Scholar]
- 19.Ayirala S, Kumar S, O’Sullivan DM, Silverman DI. Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr 2011;24:499–505. [DOI] [PubMed] [Google Scholar]
- 20.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol 1995;25:452–459. [DOI] [PubMed] [Google Scholar]
- 21.Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Lip GY, Chen SA. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol 2015;65:635–642. [DOI] [PubMed] [Google Scholar]
- 22.Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, Stollberger C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J 2004;25:1734–1740. [DOI] [PubMed] [Google Scholar]
- 23.The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998;128:639–647. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 25.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–1764. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Suwa M, Kobashi A, Yagi H, Hirota Y, Kawamura K. Influence of altered loading conditions on left atrial appendage function in vivo. Am J Cardiol 1998;81:1056–1059. [DOI] [PubMed] [Google Scholar]
- 27.Tabata T, Oki T, Iuchi A, Yamada H, Manabe K, Fukuda K, Abe M, Fukuda N, Ito S. Evaluation of left atrial appendage function by measurement of changes in flow velocity patterns after electrical cardioversion in patients with isolated atrial fibrillation. Am J Cardiol 1997;79:615–620. [DOI] [PubMed] [Google Scholar]
- 28.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–1457. [PubMed] [Google Scholar]
- 29.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994;89:224–227. [DOI] [PubMed] [Google Scholar]