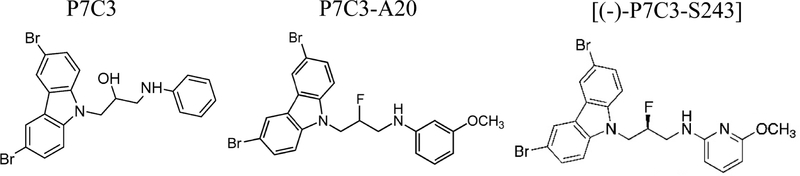
Figure 1.
Chemical structures of the P7C3 series of drug-like aminopropyl carbazoles. Chemical structure of the P7C3 parent compound (left). Highly-active P7C3–A20 (center) has fluorine substituted for the central hydroxyl and a methoxy group on the aniline ring. In the optimized derivative, (−)-P7C3–S243 (right), the aniline moiety is replaced with an alternative heterocycle conferring increased polarity. This compound can be synthesized as a single enantiomer and currently maintains the most favorable physicochemical properties.