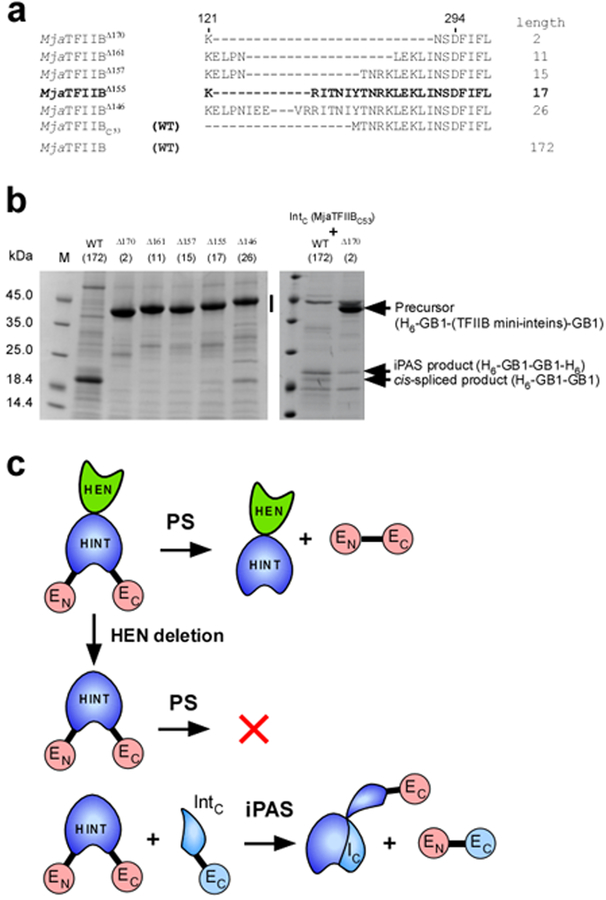
Figure 3:
SDS-PAGE analysis of cis-splicing and protein alternative splicing (iPAS) of the full-length MjaTFIIB intein and engineered MjaTFIIB mini-inteins. (a) The sequence alignment of the loop region among the engineered MjaTFIIB mini-inteins and the split intein. The numbers of amino acid residues deleted are indicated in superscript with the name of inteins. The lengths of the loop between residue 121 and 294 are indicated at the right side. WT stands for the wild-type sequence. C53 in subscript indicates the C-terminal 53 residue of the intein. (b) SDS-PAGE analysis of the elution fractions from IMAC purification using the N-terminal His-tag in the precursor protein for cis-splicing and alternative splicing. In the left panel, the vertical bar indicates the region where bands of unreacted cis-splicing precursor proteins are expected. The right panel shows SDS-PAGE analysis of the elution fractions from co-expression of the C-terminal split intein fragment (MjaTFIIBC53-GB1-H6) with the cis-splicing precursors indicated at the top. Arrows indicate, the position of a precursor protein, the band from iPAS product H6-GB1-GB1-H6, and the band corresponding to the cis-spliced product of H6-GB1-GB1. M stands for molecular weight markers. The numbers within brackets show the numbers of residues between residue 121 and 294. (c) A cartoon presentation of the effects of the HEN domain (in green) on cis-splicing by HINT domain (in blue) and co-expression of the C-terminal split intein fragment (IntC). HEN deletion results in a cis-splicing deficient intein, which can be partially activated by iPAS with an intein fragment (IC).