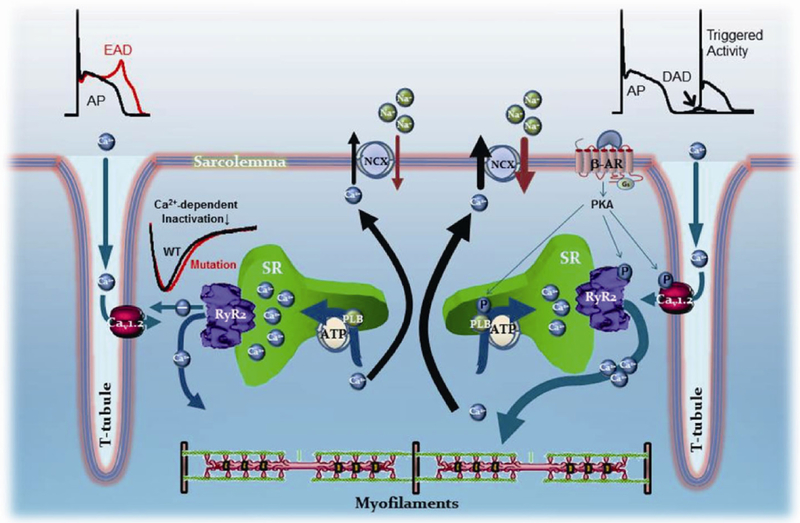
Figure 1.
Arrhyhmogenic mechanisms in ventricular myocytes harboring RyR2 mutations linked to CPVT. Left side, CPVT mutations that render RyR2 channels hypo-active or hypo-responsive to stimuli. Depolarization of T-tubules open L-type Ca2+ channels (DHPRs, or Cav1.2), which induce the opening of RyR2 channels in the SR. Ca2+ release from the SR then contributes to inactivate the L-type Ca2+ channel current (ICa). If Ca2+ release is reduced, then there is altered inactivation of ICa, prolongation of the action potential, and generation of early afterdepolarizations (EADs). Right side, The vast majority of CPVT mutations endow RyR2 channels with a gain-of-function. Stimulation of β1-adrenergic receptors by epinephrine activates PKA, which enhances Ca2+ entry and accelerates SR Ca2+ reuptake by phosphorylating L-type Ca2+ channels and phospholamban, respectively. The overall effect of sympathetic stimulation is an intracellular Ca2+ overload that increases the strength of contractions. In the process, when SR Ca2+ content is elevated, RyR2 channels hyper-sensitized by the CPVT mutations release Ca2+ spontaneously, usually in diastole, generating a depolarizing current (delayed afterdepolarization or DAD) as the released Ca2+ is extruded by the electrogenic Na+/Ca2+ exchanger. If Ca2+ released is of sufficient mass, it may generate a full action potential, which is the basis for triggered activity. See text for more details.