Abstract
Introduction
Melanoma has been reported as the most common malignancy in skin cancer. The small nucleolar RNA host gene 5 (SNHG5), an lncRNA, has been proven as a vital regulator in several types of carcinoma. This study was designed to investigate the detailed roles and possible mechanisms of SNHG5 in melanoma progression.
Methods
Quantitative real-time PCR (qRT-PCR) analysis was conducted to detect the expression levels of SNHG5, miR-26a-5p and transient receptor potential, canonical 3 (TRPC3) mRNA in melanoma tissues and cells. CCK-8 assay was used to measure the cell viability. Flow cytometry assays were performed to determine the cell cycle distribution and apoptosis. The invasive ability was assessed by a 24-well Transwell insert. Western blot analysis was employed to evaluate the protein expression of TRPC3. Dual luciferase reporter assay, RNA immunoprecipitation (RIP) assay, and RNA pull-down assay were applied to identify the interactions among SNHG5, miR-26a-5p and TRPC3.
Results
The results showed that SNHG5 expression was increased in melanoma tumor tissues and cell lines. Higher SNHG5 expression was correlated with advanced pathogenic status. Moreover, SNHG5 could serve as a molecular sponge of miR-26a-5p. SNHG5 downregulation repressed proliferation, promoted apoptosis, and decreased invasion in melanoma cells, while these effects were greatly counteracted by miR-26a-5p inhibitor. Furthermore, miR-26a-5p directly targeted TRPC3 to suppress its expression, and this effect was aggravated following SNHG5 downregulation. Also, TRPC3 depletion exerted similar tumor-suppressive functions as SNHG5 knockdown.
Conclusion
SNHG5 promoted melanoma development by inhibiting miR-26a-5p and facilitating TRPC3 expression, highlighting the potential of SNHG5 as a novel target therapy for melanoma.
Keywords: lncRNA, SNHG5, miR-26a-5p, TRPC3, cutaneum carcinoma
Introduction
Melanoma, the most common malignancy in cutaneous carcinoma, is estimated to occupŷ90% of new confirmed skin cancer cases in the USA in 2016.1 Melanoma is prone to metastasis, resulting in an unfavorable prognosis of patients in distant stage, with a 5-year survival rate of only 18%.2 Although immune-based approaches and chemoprevention have improved the management of metastatic disease, drug toxicity and resistance are still major challenges.3,4 Thus, elucidating the mechanisms underlying melanoma progression and identifying novel therapeutic targets are of great significance.
lncRNAs, defined as a kind of transcripts over 200 nucleotides in length, play important roles in human cancers by chromatin remodeling, as well as transcriptional and post-transcriptional regulation.5 Increasing lncRNAs have been found to be involved in melanoma development through modulation of different mechanisms and pathways.6 For instance, focally amplified lncRNA on chromosome 1 recruited enhancer of zeste homolog 2 to the promoter of p21, thereby repressing p21 expression and facilitated cell proliferation in melanoma.7 Depletion of urothelial carcinoma associated 1 inhibited melanoma cell proliferation and invasion via up-regulating miR-507 and decreasing forkhead box protein M1.8 Small nucleolar RNA host gene 5 (SNHG5), 524 bp in length, was initially discovered to be located on chromosome 6q15 at the chromosomal translocation breakpoint implicated in B-cell lymphoma.9 Moreover, SNHG5 was previously reported to be functionally associated with several tumors, such as chronic myeloid leukemia,10 gastric cancer,11,12 colorectal cancer,13 and bladder cancer.14 Ichigozaki et al disclosed that the serum level of SNHG5 was increased in malignant melanoma patients compared with that in normal subjects.15 Also, a recent document revealed that SNHG5 expression was upregulated in melanoma tissues and cells, and SNHG5 knockdown blocked proliferation and induced apoptosis by modulating miR-155.16 Nevertheless, the roles and molecular bases of SNHG5 in melanoma pathogenesis deserve to be further explored.
The current study elucidates a novel SNHG5/miR-26a-5p/transient receptor potential, canonical 3 (TRPC3) pathway in regulating proliferation, apoptosis, and invasion of melanoma cells, indicating that SNHG5 could serve as a candidate molecular target for melanoma treatment.
Materials and methods
Tissue specimens
Tumor tissues and adjacent noncancerous tissues were gathered from 50 cases of melanomas patients (35 males and 15 females, mean age 62.3±11.5 years) undergoing surgical resection between 2014 and 2016 at our hospital. Corresponding noncancerous tissues were obtained at least 5 cm away from the tumor sites. No patients received preoperative radiotherapy or chemotherapy. The histopathologic diagnosis was confirmed following the WHO criteria by two independent pathologists. Fresh tissue samples were immediately snap-frozen in liquid nitrogen and then stored at −80°C until RNA extraction. This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from each participant at the start of this study.
Cell culture
Melanoma cell lines (A375, SK-MEL-110, M14, MEL-RM, B16, and A2508) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and incubated in DMEM (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated FBS (Gibco-BRL). Normal human epidermal melanocytes (HEMa-LP; Invitrogen, Carlsbad, CA, USA) were maintained in Medium 254 (Cascade Biologics, Portland, OR, USA) containing human melanocyte growth supplement (Cascade Biologics). All cells were cultured in an atmosphere of 5% CO2 at 37°C.
Transfection with oligonucleotides and plasmids
The full length cDNA sequences of SNHG5 were cloned into pcDNA3.1 vector (Invitrogen) to construct SNHG5-overexpressing plasmid (pcDNA-SNHG5). SiRNA specifically against SNHG5 (si-SNHG5), siRNA specifically targeting TRPC3 (si-TRPC3) and scrambled siRNAs (si-con), miR-26a-5p mimic and its scrambled control (miR-con), as well as miR-155 inhibitor (anti-miR-155) were synthesized by GenePharma Co. Ltd. (Shanghai, China). All oligonucle-otides and plasmids were transfected into melanoma cells by lipofectamine 3000 reagent (Invitrogen) in accordance with the manufacturer’s specification.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from melanoma tissues and cells using TRIzol reagent (Invitrogen). Then, equal amount of RNA was reversely transcribed to cDNA using a Reverse Transcription Kit (Takara, Dalian, China). RT-PCR was conducted using the SYBR-Green PCR Master Mix kit (Takara) on an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The 2-ΔΔCt method was applied to measure gene expression with glyceraldehyde-3-phosphate dehydrogenase or U6 snRNA as the internal control.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 (Biotechwell, Shanghai, China) was employed to detect the proliferative ability of melanoma cells. Briefly, transfected A375 and A2508 cells (5×103 cells/well) were seeded into 96-well plates. At indicated times (24, 48, 72, and 96 hours), 10 µL of CCK-8 solution was added, and cells were further incubated for 2 hours at 37°C. A microplate reader (Thermo Labsystems, Waltham, MA, USA) was used to detect the absorbance at 450 nm.
Flow cytometry assay of cell cycle and apoptosis
Transfected A375 and A2508 cells were inoculated into 6-well plates and cultured for 48 hours. For cell cycle analysis, cells were collected, washed with PBS, fixed in 75% ethanol, incubated with 50 µg/mL propidium iodide containing 40 µg/mL RNase for 30 minutes, followed by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) to measure the distribution of cells in G0/G1, S, and G2/M phases. For apoptosis detection, cells were harvested, washed with PBS, stained with a fluorescein isothiocyanate Annexin V Apoptosis Detection Kit (BD Biosciences), and then subjected to a flow cytometer (BD Biosciences) for the examination of apoptotic rate.
Cell invasion analysis
A 24-well Transwell insert (Corning Incorporated, Corning, NY, USA) pre-coated with Matrigel of 8 µm pores was used to determine the invasive ability of melanoma cells. A375 and A2508 cells (1×105) in 100 µL serum-free medium with 1 µg/mL Mitomycin C were plated into the top chamber, and 400 µL medium with 10% FBS was added into the bottom chamber as a chemotactic agent. Following incubation of 24 hours, non-invasive cells were wiped, and cells that passed through the membrane to the lower surface were fixed with paraformaldehyde, stained with crystal violet, and counted with an inverted microscope.
Western blot analysis
Cells were collected and lysed in RIPA buffer (Sigma-Aldrich, St Louis, MO, USA). The protein lysates were separated in 10% SDS-PAGE gel and then electrophoretically transferred to polyvinylidene difluoride membranes (Roche Diagnostics, Indianapolis, IN, USA). Subsequently, the membranes were incubated with primary antibodies against TRPC3 (Abcam, Cambridge, MA, USA) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by probed with horseradish peroxidase-conjugated secondary antibody. Finally, the blots were detected and visualized with an Echo-chemiluminescence Detection System (GE Healthcare, Little Chalfont, UK).
Luciferase reporter assays
The partial sequences of SNHG5 or TRPC3-3′UTR containing predicated miR-26a-5p binding sites were amplified by PCR and then inserted into pmiRGLO reporter vector (Promega, Madison, WI, USA) to generate wild type SNHG5 reporter (SNHG5-wt) and TRPC3reporter (TRPC3-wt). Fast site-directed mutagenesis kit (Tiangen Biotech, Beijing, China) was applied to produce the mutant SNHG5 reporter (SNHG5-mut) or TRPC3reporter (TRPC3-wt) in which the binding sites were replaced. A375 cells were co-transfected with the constructed reporters and miR-con or miR-26a-5p by lipofectamine 3000 reagent (Invitrogen). After 48 hours, the relative luciferase activities were monitored by a dual-luciferase reporter assay system (Promega).
RNA immunoprecipitation (RIP) assay
EZ-Magna RIP RNA-binding protein immunoprecipitation kit (Millipore, Bedford, MA, USA) was used to perform RIP. Briefly, cell lysates were incubated with RIP buffer containing magnetic beads conjugated with argonaute-2 (Ago2) (Millipore) antibody or negative control IgG (Millipore). Immunoprecipitated RNA was purified, and then subjected to qRT-PCR analysis to detect the relative levels of SNHG5 and miR-26a-5p.
RNA pull-down assay
The pull-down assays were conducted as described in a previous document.17 Briefly, biotinylated SNHG5 was transfected into A375 cells. A biotinylated lncRNA that is not complementary to miR-26a-5p was employed as a negative control. After 48 hours, cell lysates were incubated with M-280 streptavidin magnetic beads (Invitrogen), followed by qRT-PCR assay to measure miR-26a-5p level.
Statistical analysis
All data were presented as mean ± SD and analyzed using Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Wilcoxon matched pairs test was used to examine the difference of gene expression levels between tumor and adjacent noncancerous tissues. Student’s t-test and one-way ANOVA were used to assess the significant difference between two or more groups. Pearson correlation analysis was performed to examine the correlation between SNHG5, miR-26a-5p and TRPC3. P-value <0.05 was defined as statistically significant (P<0.05, P<0.01, P<0.001).
Results
SNHG5 expression was increased in melanoma tumor tissues and cells
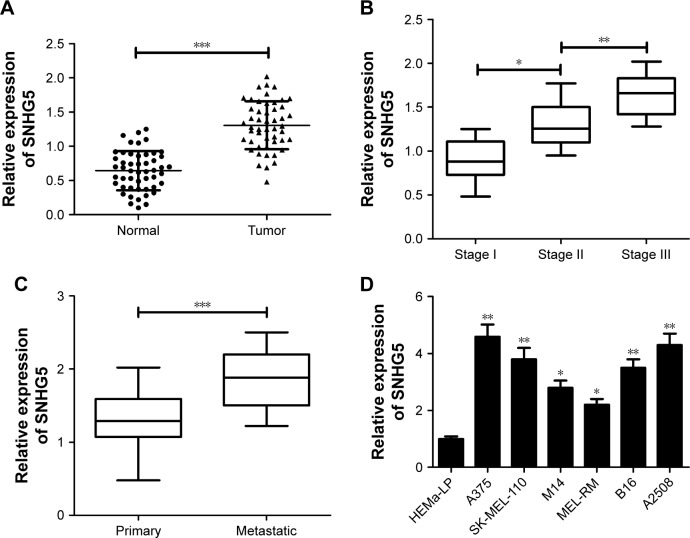
First, qRT-PCR analysis was used to measure SNHG5 expression levels of tumor tissues and corresponding non-cancerous tissues in 50 melanoma patients. Results showed that SNHG5 expression was significantly enhanced in tumor tissues compared with that in adjacent normal tissues (Figure 1A). Moreover, SNHG5 expression was upregulated with the development of pathological stage (stage I–III) (Figure 1B). Also, SNHG5 expression was found to be higher in metastatic melanoma tissues than that in primary melanoma tissues (Figure 1C). Subsequently, the expression of SNHG5 in melanoma cells (A375, SK-MEL-110, M14, MEL-RM, B16, and A2508) and normal HEMa-LP was further detected. Similar with the tissue expression, a dramatic increase in SNHG5 expression was observed in melanoma cells when compared with that in HEMa-LP cells (Figure 1D). All these data suggested that abnormal expression of SNHG5 might be associated with melanoma progression.
Figure 1.
SNHG5 expression was increased in tumor tissues and cells of melanoma.
Notes: qRT-PCR analysis of SNHG5 expression in tumor tissues and adjacent normal tissues of 50 melanoma patients (A), in different clinical pathological stages (B), in primary and metastatic melanoma tissues (C), as well as in melanoma cells (A375, SK-MEL-110, M14, MEL-RM, B16, and A2508) and normal human epidermal melanocytes (HEMa-LP) (D). *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: qRT, quantitative real-time; SNHG5, small nucleolar RNA host gene 5.
SNHG5 acted as a molecular sponge of miR-26a-5p in melanoma cells
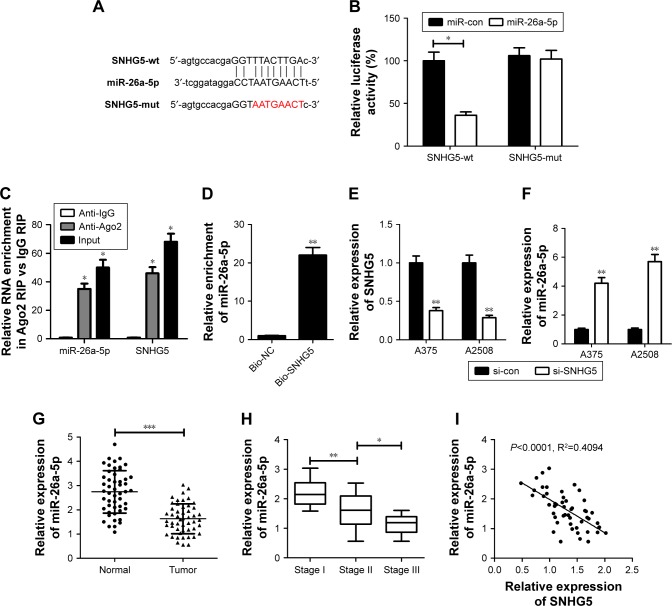
A growing body of evidence illuminates that lncRNAs could act as miRNA sponges/decoys, thereby sequester miRNAs away from target mRNAs.18 Therefore, an online software starBase version 2.0 (http://starbase.sysu.edu.cn/mirLncRNA.php) was applied to predict the possible target miRNAs of SNHG5. MiR-26a-5p was displayed to contain potential binding sites of SNHG5 (Figure 2A), and selected for further investigation for its anti-cancerous effect in several malignancies, including melanoma.19,20 Subsequently, dual luciferase reporter, RIP and RNA pull-down assays were performed in A375 cells to validate the direct interaction between SNHG5 and miR-26a-5p. Luciferase reporter experiment manifested that miR-26a-5p overexpression significantly decreased the luciferase activity of SNHG5-wt reporter, while had little effect on that of SNHG-mut reporter (Figure 2B). RIP assays uncovered that the levels of both SNHG5 and miR-26a-5p were greatly enriched by Ago2 antibody compared with control IgG (Figure 2C). RNA pull-down analysis exhibited a substantial enrichment of miR-26a-5p in the metastasis-associated lung adenocarcinoma transcript 1 pulled down pellets compared with control group (Figure 2D). Then, the expressions of SNHG5 and miR-26a-5p were determined in melanoma cells transfected with si-SNHG5. As a result, SNHG5 expression declined and miR-26a-5p expression enhanced in A375 and A2508 cells following SNHG5 knockdown (Figure 2E and F). Moreover, miR-26a-5p expression was discovered to be downregulated in melanoma tumor tissues when compared with that in corresponding noncancerous tissues (Figure 2G). Furthermore, SNHG5 expression decreased with the development of pathological stage (stage I–III) (Figure 2H). Also, SNHG5 expression was negatively correlated with SNHG5 expression in melanoma tumor tissues (Figure 2I). Collectively, SNHG5 suppressed miR-26a-5p expression as a molecular sponge in melanoma.
Figure 2.
SNHG5 suppressed miR-26a-5p expression as a molecular sponge in melanoma cells.
Notes: (A) The putative binding sites between SNHG5 and miR-26a-5p. (B) Dual luciferase reporter assays were conducted to detect the luciferase activity in A375 cells co-transfected with SNHG5-wt or SNHG5-mut reporter and miR-con or miR-26a-5p. (C) RIP assays were performed using Ago2 antibody or control IgG antibody in A375 cells, and then qRT-PCR analysis was used to measure the enrichment degrees of SNHG5 and miR-26a-5p in coprecipitated RNA. (D) RNA pull-down experiment was carried out to determine the binding between SNHG5 and miR-26a-5p in A375 cells. (E, F) qRT-PCR analysis of SNHG5 and miR-26a-5p expressions in A375 and A2508 cells after transfection with si-con or si-SNHG5. (G) MiR-26a-5p expression in tumor tissues and corresponding non-cancerous tissues of 50 melanoma patients. (H) MiR-26a-5p expression in tumor tissues at different clinical pathological stages. (I) The negative correlation between SNHG5 and miR-26a-5p expressions in 50 cases of melanoma tumor tissues. *P<0.05, **P<0.01, ***P<0.001. Bio-NC, a biotinylated lncRNA that is not complementary to miR-26a-5p was employed as a negative control.
Abbreviations: mut, mutant; qRT, quantitative real-time; RIP, RNA immunoprecipitation; si-con, scrambled siRNAs; SNHG5, small nucleolar RNA host gene 5; WT, wild type.
SNHG5 knockdown repressed proliferation, induced apoptosis, and reduced invasion in melanoma cells via regulating miR-26a-5p
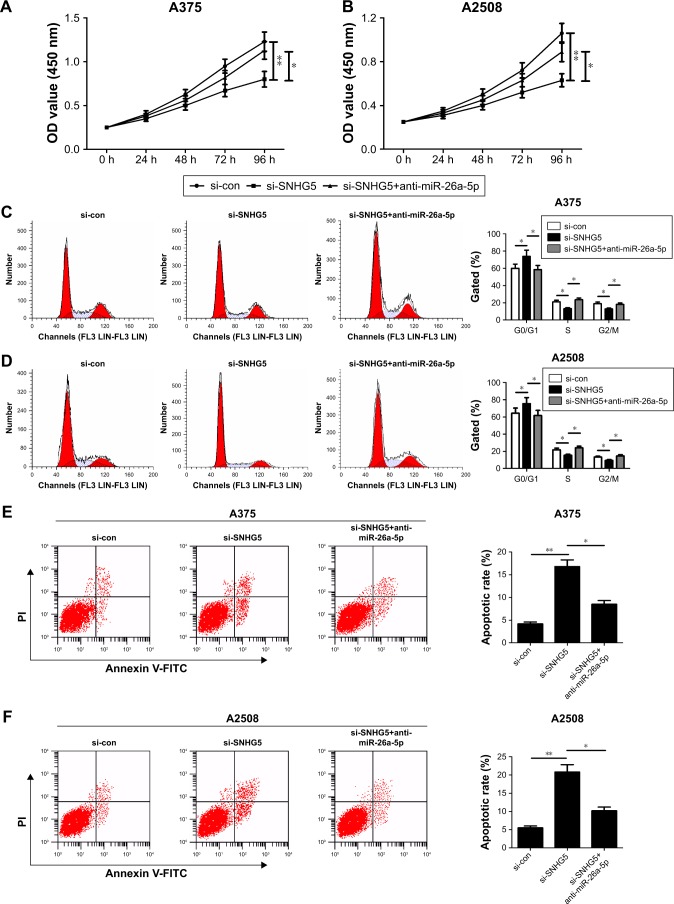
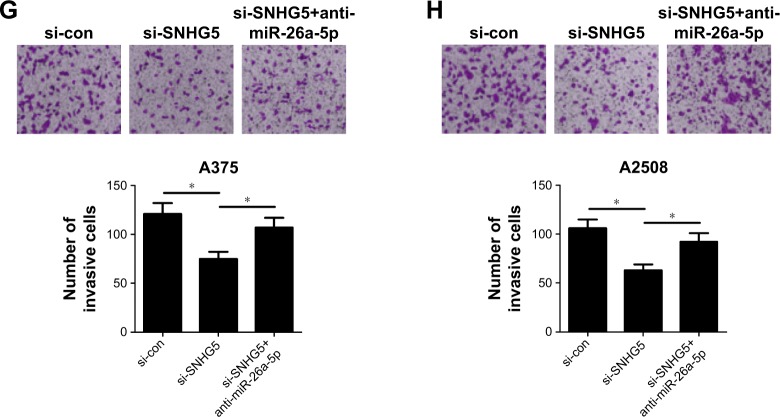
To explore the biological function and molecular mechanism of SNHG5 in melanoma, A375 and A2508 cells were transfected with either si-SNHG5 alone, or together with anti-miR-26a-5p. CCK-8 assay revealed that SNHG5 knockdown hindered proliferation ability of A375 and A2508 cells, while this effect was apparently abated after transfection with anti-miR-26a-5p (Figure 3A and B). Flow cytometry assay showed that SNHG5 downregulation triggered an obvious cell cycle arrest in A375 and A2508 cells; however, inhibition of miR-26a-5p greatly undermined this effect (Figure 3C and D). Moreover, the apoptotic rates of A375 and A2508 cells were evidently increased following depletion of SNHG5, whereas miR-26a-5p inhibitor greatly reversed si-SNHG5-induced apoptosis (Figure 3E and F). In addition, transwell invasion analysis displayed a decrease in invasive capability in si-SNHG5-transfected A375 and A2508 cells, which was remarkably abrogated by downregulation of miR-26a-5p (Figure 3G and H). Together, SNHG5 knockdown exerted tumor-suppressive function through inhibiting miR-26a-5p in melanoma.
Figure 3.
SNHG5 knockdown suppressed proliferation, induced cell cycle arrest, promoted apoptosis, and decreased invasion in melanoma cells.
Notes: A375 and A2508 cells were transfected with either si-SNHG5 alone, or together with anti-miR-26a-5p, followed by the examination of cell proliferation capability by CCK-8 (A, B), cell cycle distribution (C, D) and apoptosis (E, F) by flow cytometry analysis, as well as cell invasion ability by transwell invasion assay (G, H). *P<0.05, **P<0.01. Magnification ×200.
Abbreviations: CCK-8, Cell Counting Kit-8; FITC, fluorescein isothiocyanate; OD, optical density; PI, propidium iodide; si-con, scrambled siRNAs; SNHG5, small nucleolar RNA host gene 5; RIP, RNA immunoprecipitation.
TRPC3 mediated by SNHG5/miR-26a-5p contributed to melanoma progression
It is well known that miRNAs exert functions via directly targeting their downstream genes. Online-available tool TargetScan (http://www.targetscan.org) was applied to predict the candidate miRNAs. TRPC3, widely expressed in human melanoma, was found to possess a binding sequence within miR-26a-5p (Figure 4A). Subsequent luciferase reporter experiment showed that overexpression of miR-26a-5p significantly reduced the luciferase activity of TRPC3-wt reporter in A375 cells, which was tremendously undermined by upregulation of SNHG5 (Figure 4B). Nevertheless, no change was observed in the luciferase activity of TRPC3-mut reporter following any transfection (Figure 4B). Then, the effects of miR-26a-5p or SNHG5 on TRPC3 protein expression were evaluated in both A375 and A2508 cells. Western blot analysis manifested that either miR-26a-5p overexpression or SNHG5 knockdown resulted in a prominent decrease in TRPC3 protein level, and this effect was aggravated after co-transfection with si-SNHG5 and miR-26a-5p (Figure 4C). Moreover, TRPC3 expression was revealed to be higher in melanoma tumor tissues than that in matched normal tissues (Figure 4D). Furthermore, TRPC3 expression was found to be enhanced in tumor tissues with the development of pathological stage (stage I–III) (Figure 4E). In addition, TRPC3 expression was inversely correlated with miR-26a-5p expression (Figure 4F), and positively related to SNHG5 expression (Figure 4G). These results indicated that SNHG5 could facilitate TRPC3 expression via sponging miR-26a-5p. Subsequently, the exact roles of TRPC3 in melanoma were further investigated in A375 cells by introducing si-TRPC3. As expected, TRPC3 expression was significantly reduced following si-TRPC3 transfection (Figure 4H). The follow-up function assays elucidated that TRPC3 knockdown distinctly suppressed proliferation (Figure 4I), induced cell cycle arrest (Figure 4J), enhanced apoptosis (Figure 4K), and decreased invasion (Figure 4L). Summarily, the tumor-suppressive effect of TRPC3 knockdown was mediated by SNHG5/miR-26a-5p.
Figure 4.
The tumor-suppressive roles of TRPC3 silencing were mediated by SNHG5/miR-26a-5p.
Notes: (A) The predicted complementary sequence between miR-26a-5p and TRPC3-3′UTR region. (B) Luciferase activity was evaluated in A375 cells co-transfected with TRPC3-wt or TRPC3-mut reporter and miR-con, miR-26a-5p, miR-26a-5p+vector, or miR-26a-5p+pcDNA-SNHG5. (C) Western blot analysis was performed in A375 and A2508 cells to assess the effect of miR-26a-5p overexpression or SNHG5 knockdown on TRPC3 expression. Every experiment was performed in triplicate and repeated three times. (D) TRPC3 expression in tumor tissues and adjacent normal tissues of 50 melanoma patients. (E) TRPC3 mRNA expression in tumor tissues at different clinical pathological stages. (F, G) The correlation assay between TRPC3 and miR-26a-5p or SNHG5 in 50 cases of melanoma tumor tissues. A375 cells were transfected with si-con or si-TRPC3, followed by qRT-PCR analysis of TRPC3 expression (H), MTT analysis of cell proliferation capability (I), flow cytometry assay of cell cycle distribution (J) and apoptotic rate (K), as well as transwell invasion assay of invasion ability (L). *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: FITC, fluorescein isothiocyanate; mut, mutant; miR-con, scrambled miRNA control; OD, optical density; qRT, quantitative real-time; si-con, scrambled siRNAs; SNHG5, small nucleolar RNA host gene 5; TRPC3, transient receptor potential, canonical 3; WT, wild type.
Discussion
lncRNAs have been discovered to be associated with the pathogenesis of cutaneous melanoma via modulating various pathways and molecular interactions.21,22 This study is aimed at investigating the detailed roles and underlying mechanisms of SNHG5 in melanoma.
SNHG5 functions as a cancer-causing oncogene or tumor suppressor in different types of cancers. For example, SNHG5 overexpression suppressed gastric cancer cells proliferation and migration via regulating miR-32/Kruppel-like factor 4 pathway.11 Also, SNHG5 expression was decreased in gastric cancer tissues, and SNHG5 upregulation blocked cell proliferation and metastasis through trapping metastasis-associated protein 2 in the cytosol.12 Conversely, SNHG5 expression was increased in colorectal cancer, and SNHG5 promoted colorectal cancer cell survival by alleviating Staufen 1-induced degradation of spermatogenesis-associated serine-rich protein 2.13 Additionally, SNHG5 expression was upregulated in bladder cancer, and SNHG5 knockdown repressed cell growth and induced apoptosis in a p27-dependent manner.14 In the current study, SNHG5 expression was found to be upregulated in melanoma tumor tissues and cell lines, and correlated with advanced pathological stages, consistent with a previous study.16
In recent years, accumulating evidence elucidates that lncRNAs can act as miRNA sponges to reduce the amount of miRNAs available to target mRNAs.23 Therefore, the possible regulatory pathways of SNHG5 in melanoma were further explored. By using bioinformatic tools, miR-26a-5p was discovered to harbor a complementary sequence in SNHG5. MiR-26a-5p has been revealed to impair cell aggressiveness in several malignant tumors. For instance, Miyamoto et al reported that miR-26a-5p expression was downregulated in bladder cancer, and miR-26a-5p overexpression repressed cell migration and invasion by regulating procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2.24 Chang et al confirmed a strong reduction of miR-26a-5p expression in hepatocellular carcinoma, and inhibition of miR-26a-5p promoted cell invasion and metastasis via modulating epithelial–mesenchymal transition.25 Tormo et al delineated that miR-26a overexpres-sion sensitized HER2+ breast cancer cells to trastuzumab treatment by affecting cyclin E2 (CCNE2) gene.26 Interestingly, miR-26a-5p expression was found to be reduced in melanoma,27 and enforced expression miR-26a suppressed the growth and invasiveness of melanoma cells by targeting microphthalmia-associated transcription factor.20 Subsequent luciferase reporter, RIP and RNA pull-down experiments verified that SNHG5 was able to directly bind to miR-26a-5p. Moreover, miR-26a-5p expression was promoted in both A375 and A2508 cells after transfection with si-SNHG5. Taken together, SNHG5 could inhibit miR-26a-5p expression by direct interaction. Then, whether SNHG5 exerts biological functions in melanoma by sponging miR-26a-5p was analyzed. Function assay experiments demonstrated that depletion of SNHG5 triggered a great diminishment in cell growth, cell cycle progression, and invasion, while an obvious enhancement of apoptosis. However, the tumor-suppressive effects mediated by SNHG5 silence were substantially reversed following inhibition of miR-26a-5p. These data uncovered that SNHG5 exerted functions as an oncogene in melanoma by sponging miR-26a-5p. Coincident with our results, Yan et al validated that SNHG5 knockdown suppressed proliferation and facilitated apoptosis in melanoma cells by serving as a molecular sponge of miR-155.16
Next, the TargetScan software and luciferase reporter assay disclosed TRPC3 as a direct target of miR-26a-50p. Abnormal regulation of TRP ion channel gating or expres sion levels leads to deregulation of downstream effectors sensitive to changes in Ca2+ homeostasis, thereby promoting pathophysiological cancer hallmarks, such as increased survival, proliferation, and invasion.28 TRPC3, one of the classical members of mammalian TRP superfamily of ion channels, has been reported to participate in the pathogenesis in certain cancers.29 For example, knockdown of TRPC3 led to severe decrease in cell proliferation and invasion ability in bladder cancer.30 Downregulation of TRPC3 caused by TRPC channel blockers or siRNA targeting TRPC3 repressed cell proliferation, while increase in TRPC channel activity and TRPC3 overexpression contributed to cell growth in ovarian cancer.31 In this study, it was further found that SNHG5 could sponge miR-26a-5p, thereby derepressing the expression TRPC3. Loss-of-function experiments revealed that suppression of TRPC3 expression weakened proliferation, enhanced cell cycle arrest, facilitated apoptosis, and lowered invasion of melanoma cells. In agreement with our results, pharmacological inhibition of TRPC3 or silencing of TRPC3 gene expression effectively inhibited growth and migration of melanoma cells through activation of Akt and STAT pathways.32 A previous report revealed that TRPC3 channel blocker Pyr3 induced dexamethasone sensitivity and enhanced apoptosis in acute lymphoblastic leukemia cells by disturbing Ca2+ signaling.33 However, the effect of TRPC3 on the ion channel activity in melanoma needs to be further investigated in future.
Conclusion
In summary, our present study proved that SNHG5 contributed to melanoma development possibly by sponging miR-26a-50p and increasing TRPC3 expression, suggesting that targeting SNHG5/miR-26a-50p/TRPC3 axis may be a promising therapy strategy for melanoma patients. However, the regulatory networks of lncRNAs in cancers are complicated. Thereby, more mechanistic researches and animal experiments are required for better elaborating SNHG5 in melanoma.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;6666(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;6767(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Luke JJ, Schwartz GK. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin Dermatol. 2013;31(3):290–297. doi: 10.1016/j.clindermatol.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boada A, Carrera C, Segura S, et al. Cutaneous toxicities of new treatments for melanoma. Clin Transl Oncol. 2018;20(11):1373–1384. doi: 10.1007/s12094-018-1891-7. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richtig G, Ehall B, Richtig E, Aigelsreiter A, Gutschner T, Pichler M. Function and clinical implications of long non-coding RNAs in melanoma. Int J Mol Sci. 2017;18(4):715. doi: 10.3390/ijms18040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni N, Song H, Wang X, Xu X, Jiang Y, Sun J. Up-regulation of long noncoding RNA FALEC predicts poor prognosis and promotes melanoma cell proliferation through epigenetically silencing p21. Biomed Pharmacother. 2017;96:1371–1379. doi: 10.1016/j.biopha.2017.11.060. [DOI] [PubMed] [Google Scholar]
- 8.Wei Y, Sun Q, Zhao L, et al. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol. 2016;33(8):88. doi: 10.1007/s12032-016-0804-2. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Takahashi N, Kakegawa E, et al. The GAS5 (growth arrest-specific transcript 5) gene fuses to BCL6 as a result of t(1;3) (q25;q27) in a patient with B-cell lymphoma. Cancer Genet Cytogenet. 2008;182(2):144–149. doi: 10.1016/j.cancergencyto.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.He B, Bai Y, Kang W, Zhang X, Jiang X. LncRNA SNHG5 regulates imatinib resistance in chronic myeloid leukemia via acting as a CeRNA against MiR-205-5p. Am J Cancer Res. 2017;7(8):1704–1713. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Han T, Li Y, et al. The lncRNA SNHG5/miR-32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. Faseb J. 2017;31(3):893–903. doi: 10.1096/fj.201600994R. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Guo H, Zhou B, et al. Long non-coding RNA SNHG5 suppresses gastric cancer progression by trapping MTA2 in the cytosol. Oncogene. 2016;35(44):5770–5780. doi: 10.1038/onc.2016.110. [DOI] [PubMed] [Google Scholar]
- 13.Damas ND, Marcatti M, Côme C, et al. SNHG5 promotes colorectal cancer cell survival by counteracting STAU1-mediated mRNA destabilization. Nat Commun. 2016;7:13875. doi: 10.1038/ncomms13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, Xue S, Zeng B, Qiu D. lncRNA SNHG5 is associated with poor prognosis of bladder cancer and promotes bladder cancer cell proliferation through targeting p27. Oncol Lett. 2018;15(2):1924–1930. doi: 10.3892/ol.2017.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichigozaki Y, Fukushima S, Jinnin M, et al. Serum long non-coding RNA, snoRNA host gene 5 level as a new tumor marker of malignant melanoma. Exp Dermatol. 2016;25(1):67–69. doi: 10.1111/exd.12868. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Wang S, Li Y, et al. SNHG5 promotes proliferation and induces apoptosis in melanoma by sponging miR-155. RSC Adv. 2018;8(11):6160–6168. doi: 10.1039/c7ra12520h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Rivetti di Val Cervo P, Lena AM, Nicoloso M, et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109(4):1133–1138. doi: 10.1073/pnas.1112257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34(4):9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo K, Zheng S, Xu Y, Xu A, Chen B, Wen Y. Loss of miR-26a-5p promotes proliferation, migration, and invasion in prostate cancer through negatively regulating SERBP1. Tumour Biol. 2016;37(9):12843–12854. doi: 10.1007/s13277-016-5158-z. [DOI] [PubMed] [Google Scholar]
- 20.Qian H, Yang C, Yang Y. MicroRNA-26a inhibits the growth and invasiveness of malignant melanoma and directly targets on MITF gene. Cell Death Discov. 2017;3:17028. doi: 10.1038/cddiscovery.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulstaert E, Brochez L, Volders PJ, Vandesompele J, Mestdagh P. Long non-coding RNAs in cutaneous melanoma: clinical perspectives. Oncotarget. 2017;8(26):43470–43480. doi: 10.18632/oncotarget.16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leucci E, Coe EA, Marine JC, Vance KW. The emerging role of long non-coding RNAs in cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29(6):619–626. doi: 10.1111/pcmr.12537. [DOI] [PubMed] [Google Scholar]
- 23.Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto K, Seki N, Matsushita R, et al. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br J Cancer. 2016;115(3):354–363. doi: 10.1038/bjc.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Li K, Guo T. miR-26a-5p suppresses tumor metastasis by regulating EMT and is associated with prognosis in HCC. Clin Transl Oncol. 2017;19(6):695–703. doi: 10.1007/s12094-016-1582-1. [DOI] [PubMed] [Google Scholar]
- 26.Tormo E, Adam-Artigues A, Ballester S, et al. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Sci Rep. 2017;7:41309. doi: 10.1038/srep41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozubek J, Ma Z, Fleming E, et al. In-depth characterization of microRNA transcriptome in melanoma. PLoS One. 2013;8(9):e72699. doi: 10.1371/journal.pone.0072699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapovalov G, Ritaine A, Skryma R, Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin Immunopathol. 2016;38(3):357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 29.Tiapko O, Groschner K. TRPC3 as a target of novel therapeutic interventions. Cells. 2018;7(7):83. doi: 10.3390/cells7070083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JM, Heo K, Choi J, Kim K, An W. The histone variant MacroH2A regulates Ca(2+) influx through TRPC3 and TRPC6 channels. Oncogenesis. 2013;2(10):e77. doi: 10.1038/oncsis.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng B, Yuan C, Yang X, Atkin SL, Xu SZ. TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets. 2013;13(1):103–116. [PubMed] [Google Scholar]
- 32.Oda K, Umemura M, Nakakaji R, et al. Transient receptor potential cation 3 channel regulates melanoma proliferation and migration. J Physiol Sci. 2017;67(4):497–505. doi: 10.1007/s12576-016-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdoul-Azize S, Buquet C, Vannier JP, Dubus I. Pyr3, a TRPC3 channel blocker, potentiates dexamethasone sensitivity and apoptosis in acute lymphoblastic leukemia cells by disturbing Ca(2+) signaling, mitochondrial membrane potential changes and reactive oxygen species production. Eur J Pharmacol. 2016;784:90–98. doi: 10.1016/j.ejphar.2016.05.014. [DOI] [PubMed] [Google Scholar]