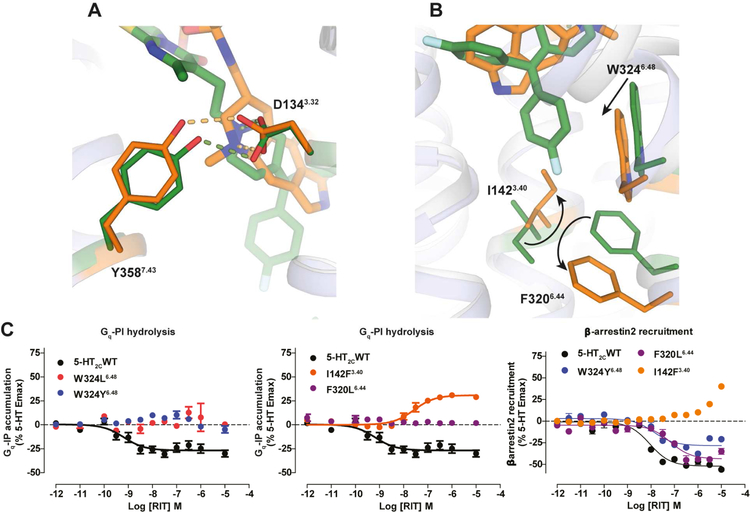
Figure 3. Conformational Changes between 5-HT2C-ERG and -RIT Structures and Mutagenesis Validation.
(A) ERG (orange sticks) and RIT (green sticks) in the binding pocket of 5-HT2C. Key residues in 5-HT2C-ERG and 5-HT2C-RIT are shown in orange and green sticks, respectively. Hydrogen bonds between D1343.32 and Y3587.43 are shown in dash line. (B) Conformational changes of I1423.40 and F3206.44 in the P-I-F motif and the W3246.48 “toggle switch” in helix VI. (C) Mutations of W3246.48 and F3206.44 completely abolish RIT’s Gαq inverse agonism, yet retain β-arrestin2 inverse agonism. Mutations of I1423.40 selectively abolish RIT’s Gαq inverse agonism and instead show weak agonism. Data represent mean ± S.E.M. from three independent experiments performed in triplicate. See also Figure S3.