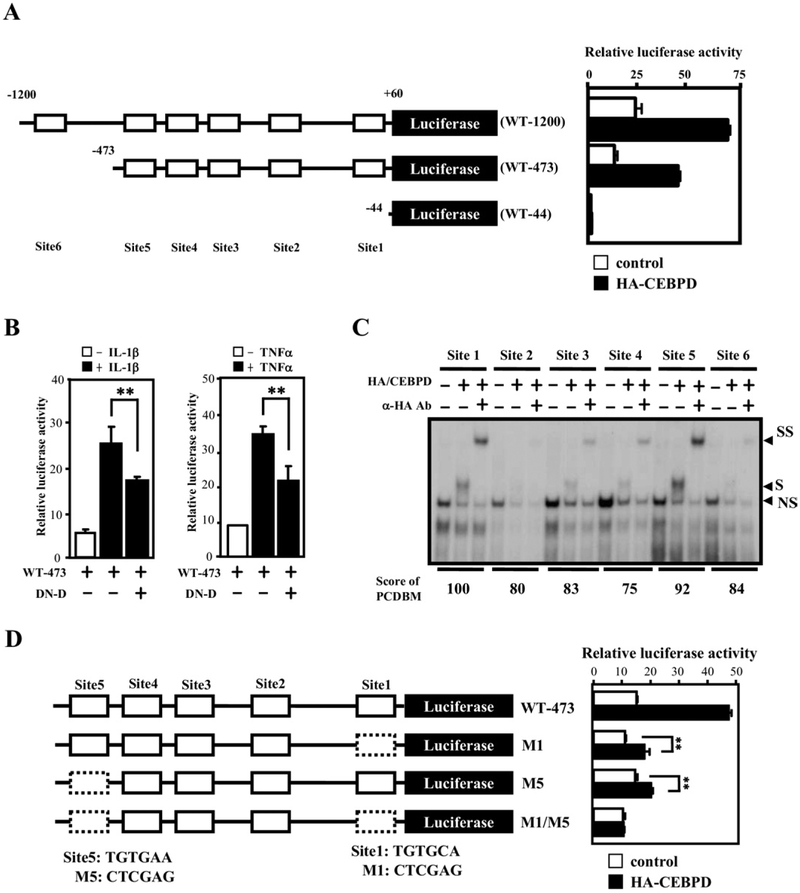
Fig. 5.
CEBPD-binding motifs are important for CEBPD-induced PTX3 reporter activity. (A) Schematic representation of reporter constructs with the human PTX3 promoter (left panel). Numbers indicate the number of base pairs upstream (−) and downstream (+) of the PTX3 translation start site. The approximate location of putative CEBPD-binding motifs (site 1–6) is indicated by open boxes. Luciferase activity from these reporter constructs in U373MG cells 12 hours after cotransfected with CEBPD expression or control vectors is shown on the right (mean ± SD, n = 3). (B) A dominant-negative CEBPD protein attenuates tumor necrosis factor alpha (TNF-α)- and interleukin (IL)-1β-activated PTX3 reporter activities. The PTX3 reporter, PTX3–473, was cotransfected with a dominant-negative CEBPD expression vector (DN-D) into U373MG cells. Lysates of the transfectants were harvested for luciferase assay 12 hours after transfection (mean ± SD, n = 3, ** p < 0.01 by Student t test). (C) CEBPD protein binds sites 1 and 5 with highest affinity. An electrophoretic mobility shift assay was performed with 32P-labeled probes bearing the putative CEBPD motifs at sites 1–6 of the PTX3 promoter (see panel (A), in vitro-translated HA-CEBPD protein, and specific CEBPD antibodies as indicated. “NS” indicates a nonspecific binding complex, “S” indicates the specific CEBPD-DNA complex, and “SS” indicates the supershift with the CEBPD antibody. (D) Both sites 1 and 5 of the PTX3 promoter are important for regulation by CEBPD. Schematic representation of the PTX3 reporters with single or double mutations (dashed boxes) in CEBPD-binding sites (left panel) cotransfected with CEBPD into U373MG cells. Lysates were prepared 12 hours after transfection and assayed for luciferase activity (right panel) (mean ± SD, n = 3, ** p <0.01 by Student t test).