Although several lines of evidence suggest that the glycosyl phosphatidylinositol-anchored cell surface protein Sca-1 marks cardiac-resident stem cells, a critical analysis of the literature raises some concerns regarding their cardiomyogenic potential.1 Here, isolated adult cardiac-resident Sca-1+ cells were engrafted into infarcted hearts and monitored for cardiomyogenic differentiation. Donor cells were prepared from ACT-EGFP; MHC-nLAC double-transgenic mice ([C57/Bl6J x DBA/2J]F1 genetic background; all procedures followed were in accordance with Institutional Guidelines). The ACT-EGFP transgene targets ubiquitous expression of an enhanced green fluorescent protein reporter, and the MHC-nLAC transgene targets cardiomyocyte-restricted expression of a nuclear-localized β-galactosidase reporter. Donor cell survival was monitored via EGFP fluorescence, while cardiomyogenic differentiation was monitored by reacting with the chromogenic β-galactosidase substrate 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-GAL), which gives rise to a blue product.2 Double-transgenic hearts were dispersed with Blendzyme and the resulting cells reacted with an APC-conjugated anti-Sca-1 antibody and a PE-conjugated cocktail of antibodies recognizing hematopoietic lineage markers.3 Sca-1+, EGFP+, lineage- cells were then isolated via fluorescence-activated cell sorting (FACS; characterization of the donor cells is provided in Figure 1A), and 100,000 cells were injected into the infarct border zone of non-transgenic [C57/Bl6J x DBA/2J]F1 mice immediately following permanent coronary artery occlusion.
Figure 1.
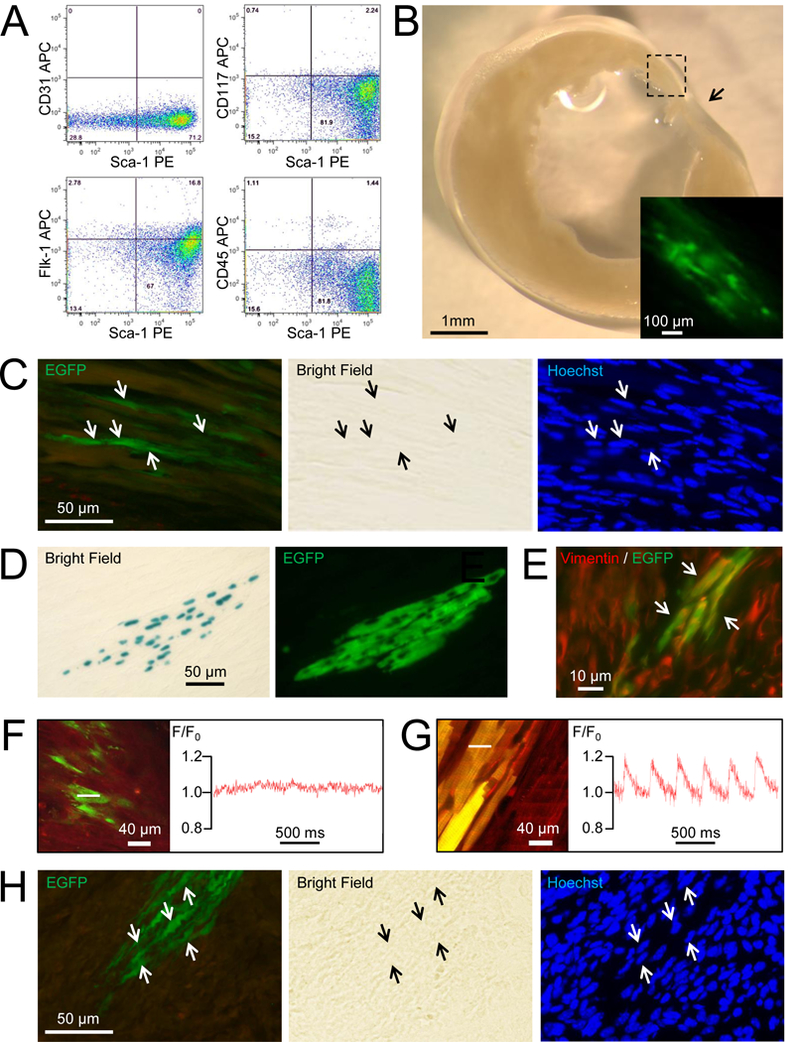
Adult cardiac-resident Sca-1+ cells fail to undergo cardiomyogenic differentiation following engraftment into infarcted mouse hearts. (A) FACS characterization of donor Sca-1+, EGFP+, lineage- donor cells isolated from adult ACT-EGFP; MHC-nLAC double-transgenic mice prior to engraftment. (B) Bright field image of a vibratome section from an infarcted heart engrafted with Sca-1+, EGFP+, lineage- cells isolated from ACT-EGFP; MHC-nLAC double-transgenic donors (the section was reacted with X-GAL). Arrow indicates the position of the infarct and the inset shows a fluorescent view of the engrafted region (box) at higher magnification. The example shown is at 14 days post-engraftment. (C) EGFP fluorescence (left panel), bright field (center panel) and Hoechst fluorescence (right panel) views of a 10 micron cryosection prepared from a vibratome section of a heart engrafted with Sca-1+, EGFP+, lineage- cells (the vibratome section was reacted with X-GAL). The donor cell nuclei (arrows) do not react with X-GAL. The example shown is at 11 days post-engraftment. (D) Bright field (left panel) and EGFP fluorescence (right panel) views of a 10 micron cryosection prepared from a vibratome section of an infarcted heart engrafted with embryonic cardiomyocytes from ACT-EGFP; MHC-nLAC double-transgenic donors (the vibratome section was reacted with X-GAL; please note that the X-GAL reaction product blocks visualization of EGFP and Hoechst fluorescence). The example shown is at 14 days post-engraftment. (E) Color combined fluorescence view of vimentin immune reactivity (red signal) and EGFP (green) in an infarcted heart engrafted with Sca-1+, EGFP+, lineage- donor cells. Yellow signal (arrows) indicates that some of the donor cells are adopting a fibroblast-like phenotype. The example shown is at 11 days post-engraftment. (F & G) In situ imaging of [Ca2+]i transients from an infarcted heart engrafted with adult Sca-1+, EGFP+, lineage- donor cells (F) or fetal cardiomyocytes from double-transgenic ACT-EGFP; MHC-nLAC transgenic mice (G). In each case, the left panels show the full-frame two-photon laser scanning image of the analyzed cells, while the right panels show the integrated traces of rhod-2 (red lines) fluorescence collected in line-scan mode; the white dotted lines indicate the positions where the integrated traces were recorded. The examples shown are at 11 days post-engraftment. The in situ imaging protocol has been described previously.4 Briefly, Langendorff-perfused hearts were loaded with the fluorescent calcium indicator rhod-2 using the cell membrane permeable AM-ester of the dye (10 μmol/L rhod-2-AM for 10 min.). Hearts were illuminated (810 nm using a mode-locked Ti:sapphire laser) and imaged with a Bio-Rad MRC 1024 laser scanning microscope; emitted light was collected at 560–650 nm and 500–550 nm). In all cases the hearts were subjected to electrical field stimulation to ensure donor cell membrane depolarization during imaging. For full-frame mode, lines were scanned at 1.46 and 0.73 frames per second on horizontal (x, y) planes. For line-scan mode, lines were scanned at 500 Hz and images (x, t) were constructed by stacking all lines vertically. (H) EGFP fluorescence (left panel), bright field (center panel) and Hoechst fluorescence (right panel) views of a 10 micron cryosection prepared from a vibratome section of a heart engrafted with Sca-1, lineage- cells from ACT-EGFP; MHC-nLAC double-transgenic donors sorted independently of EGFP expression (the vibratome section was reacted with X-GAL). The donor cell nuclei do not react with X-GAL. Example shown is at 14 days post-engraftment.
To screen for cardiomyogenic activity, hearts were harvested at 11 (n=3), 12 (n=3), 14 (n=2) or 21 (n=2) days post-engraftment, fixed and sectioned on a vibratome at 200 microns; sections were then reacted with X-GAL and visualized on a dissecting microscope. No nuclear β-galactosidase activity was detected (Figure 1B), despite the presence of Sca-1+ donor cells as evidenced by the presence of EGFP fluorescence (inset). To confirm the absence of cardiomyogenic differentiation, all vibratome sections exhibiting EGFP fluorescence were cryosectioned at 10 microns and analyzed (example shown in Figure 1C). No nuclear β-galactosidase activity was observed (8,577 EGFP+ cells analyzed, the engraftment efficiency was 0.87%). Immune cytology confirmed that the green fluorescent signal co-localized with EGFP protein (not shown). As a positive control for the reporter system, cardiomyocytes prepared from embryonic day 15.5 ACT-EGFP; MHC-nLAC double transgenic mice were engrafted into the hearts of syngeneic [C57/Bl6J x DBA/2J]F1 recipients; the hearts were harvested at 14 days post-engraftment and processed exactly as described above. Donor cells with nuclear β-galactosidase activity and EGFP fluorescence were readily observed in the engrafted hearts (Figure 1D). Immune histology revealed that at least a portion of the Sca-1+ donor cells express vimentin (Figure 1E), suggesting that they may give rise to fibroblast-like cells when engrafted into injured hearts.
Prior to fixation, all of the hearts analyzed above were screened for the presence of intracellular calcium ([Ca2+]i) transients in engrafted Sca-1+ cells. To accomplish this, hearts were perfused with cytochalasin D (to effect excitation/contraction un-coupling) and loaded with rhod-2 (an indicator dye which exhibits increased fluorescence with increased [Ca2+]i). The hearts were imaged via two-photon laser scanning microscopy to monitor the presence of EGFP and rhod-2 fluorescence.4 Donor Sca-1+, EGFP+, lineage- cells were readily detected via two-photon laser scanning microscopy due to their EGFP fluorescence (Figure 1F, left panel). Integrated traces of line-scan signals revealed the absence of transient increases in rhod-2 fluorescence, indicative of the absence of stimulation-evoked increases in [Ca2+]i (Figure 1F, right panel; 370 EGFP+ cells analyzed, n=5 hearts analyzed at 11 (n=3), 12 (n=1) and 21 (n=1) days post-engraftment). In contrast, transient changes in rhod-2 fluorescence, indicative of [Ca2+]i transients, were observed in engrafted EGFP-expressing fetal cardiomyocytes (Figure 1G, full-frame image on the left, integrated traces of rhod-2 fluorescence on the right). Collectively these data indicate that the engrafted Sca-1+, EGFP+, lineage- cells failed to undergo cardiomyogenic differentiation.
Although EGFP fluorescence is widespread in ACT-EGFP mice, expression is not 100% penetrant. To rule out the possibility that the reporter failed to mark cardiomyogenic progenitors, Sca-1+, lineage- donor cells were isolated via FACS (i.e., sorting independently of EGFP expression) from double-transgenic mice, and 100,000 cells were transplanted into the infarct border zone immediately following permanent coronary artery occlusion. No cardiomyogenic events, as evidenced by the lack of nuclear β-galactosidase activity, were detected at 14 days post-engraftment (Figure 1H; 9,319 EGFP+ cells were present in the sections analyzed, n=3 engrafted hearts; the engraftment efficiency was 3.1%). Although unlikely, the process of FACS enrichment may have selectively eliminated cells with cardiomyogenic potential. Accordingly, Sca-1+ cells from ACT-EGFP; MHC-nLAC double transgenic animals were isolated via magnetic-activated cell sorting5 and transplanted into the infarct border zone. Once again, cells with robust EGFP fluorescence were detected in the engrafted hearts (analyzed at 14 (n=13) or 21 (n=4) days post-engraftment), indicating donor cell survival. However, no cardiomyogenic events were detected (70,166 EGFP+ cells analyzed, n=17 engrafted hearts, the engraftment efficiency was 4.2%).
In total, no cardiomyogenic events were detected when 88,062 EGFP+ donor cells were analyzed amongst 30 engrafted hearts between 11 and 21 days post-engraftment; thus, adult cardiac-resident cells expressing the cell surface marker Sca-1 appear to lack cardiomyogenic activity.
Acknowledgements:
We thank Dorothy Field for excellent technical assistance with the studies.
Funding Sources:
This work was funded by National Institutes of Health grants HL132927, HL134599 and HL075165.
Footnotes
Data Sharing: The authors declare that all supporting data are available within the article
Disclosure:
None.
References:
- 1.Valente M, Nascimento DS, Cumano A and Pinto-do OP. Sca-1+ cardiac progenitor cells and heart-making: a critical synopsis. Stem Cells Dev. 2014;23:2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA and Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. [DOI] [PubMed] [Google Scholar]
- 3.Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA and Srour EF. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubart M, Soonpaa MH, Nakajima H and Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaruba MM, Soonpaa M, Reuter S and Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


