Abstract
We have previously reported that Chlamydia trachomatis plasmid-encoded Pgp3 is able to neutralize anti-chlamydial activity of human cathelicidin peptide LL-37 by binding to and forming stable complex with LL-37. Besides its microbicidal activity, LL-37 also modulates immune response, including inducing cytokine/chemokine production in fibroblast/epithelial cells and recruitment of inflammatory cells. We now report that LL-37 was significantly induced in the genital tracts of women diagnosed positive for C. trachomatis. Both the LL-37-stimulated IL-6/8 production in human endometrial epithelial cells and the LL-37-induced neutrophil chemotaxis were blocked by Pgp3. Interestingly, although Pgp3 itself alone could not induce cytokines in epithelial cell cells, it did so in neutrophils. Importantly, the Pgp3 proinflammatory activity in neutrophils was significantly enhanced by forming complex with LL-37 although LL-37 alone failed to induce cytokine production in neutrophils. Thus, we have demonstrated that Pgp3 can modulate the proinflammatory activities of LL-37 on epithelial cells by forming stable complex with LL-37 but the Pgp3’s own proinflammatory activity on myeloid cells is enhanced by forming the same complex. We hypothesize that Chlamydia may use Pgp3 to both block detrimental inflammation for improving its own fitness in the genital tract epithelial tissue and activate myeloid cell-mediated inflammation for potentially promoting spreading between the hosts, the latter of which may inevitably contribute to the development of inflammatory sequelae such as tubal fibrosis.
Keywords: Pgp3, LL-37, antimicrobial peptides, chemotaxis, cytokines, fibrosis
1. Introduction
Chlamydia trachomatis is a leading cause of sexually transmitted infections in women’s lower genital tracts [1], which may ascend to the upper genital tracts and induce inflammatory pathologies there, resulting in complications such as ectopic pregnancy and tubal factor infertility [2]. However, the mechanism by which C. trachomatis induces upper genital tract pathology remains to be elucidated. Chlamydia organisms share a highly conserved cryptic plasmid that encodes 8 open reading frames (ORFs), designated pORF1-8 or Pgp1-8 [3]. Although the cryptic plasmid is not required for chlamydial infection and replication in cell cultures, plasmid-free Chlamydiae are no longer able to induce pathology in the upper genital tract [4, 5] or ocular [6] tissues, suggesting that the plasmid must encode virulence factors that contribute to chlamydial pathogenicity in vivo.
We have previously shown that Pgp3, one of the 8 ORFs encoded by the cryptic plasmid, is secreted out of the chlamydial organisms and into chlamydial inclusion and host cell cytosol [7]. The native Pgp3 is a stable trimer and is abundantly accumulated in the infected cell cytosol at the late stages of infection [8–10]. Lysis of the infected cells may rapidly release the cytosolic Pgp3 into extracellular environment, which may make Pgp3 highly immunogenic during chlamydial infection [8, 11]. We found that Pgp3 effectively neutralized antichlamydial activity of LL-37, a cathelicidin antimicrobial peptide, by forming stable complex [12]. The Pgp3 middle region was found to be responsible for both the high affinity binding to LL-37 and the profound neutralization of LL-37 antichlamydial activity.
Besides its antimicrobial activity, LL-37 is also known to activate inflammatory pathways, stimulate epithelial cells to secrete pro-inflammatory cytokines and recruit inflammatory cells [13]. In the current study, after we demonstrated that LL-37 was indeed induced in women’s genital tracts during C. trachomatis infection, we evaluated the impacts of the plasmid-encoded Pgp3 on the proinflammatory activities of LL-37. We found that Pgp3 prevented LL-37 from inducing cytokine production in human endometrial epithelial cells and blocked LL-37 from recruiting neutrophils, which may delay the onset of a full-scale inflammatory response. Interestingly, although Pgp3 failed to activate epithelial cells, it was able to stimulate myeloid cells to secrete inflammatory cytokines [7] and the proinflammatory activity of Pgp3 on neutrophils was significantly enhanced by LL-37, which may benefit Chlamydia spreading as proposed previously [14]. Thus, the plasmid-encoded Pgp3 may exert its virulence effects by both blocking the anti-chlamydial and proinflammatory activities of LL-37 to promote chlamydial survival and using LL-37 to enhance its own proinflammatory activity for aiding chlamydial spreading.
2. Materials and Methods
2.1. Expression and purification of Pgp3
The pgp3 gene (also referred to as pORF5) encoded in pCHL1 plasmid of C. trachomatis serovar D organisms [15] was cloned into pET30a vector (Novagen, Madison, WI) and expressed as fusion protein with His-tag fused to the C-terminus [10]. The His-tagged Pgp3 was purified using nickel agarose beads (Cat#32050, Qiagen, Valencia, CA) and further washed off excessive imidazole using Centricons (Millipore, Billerica, MA). All purified proteins were concentrated and quantitated using Bio-Rad Protein Assay Dye reagent (cat# 500-0006, Bio-Rad, Hercules, CA). The protein purity was evaluated in polyacrylamide gels using commassie blue staining as described previously [7, 12].
2.2. Generation of Bone marrow-derived neutrophils
Bone marrow cells were sterilely harvested from tibiae and femurs of C3H/HeJ mice (stock#000659, The Jackson laboratory, Bar Harbor, Maine; Resistance to lipopolysaccharide or LPS due to a spontaneous mutation in toll-like receptor 4 or tlr4 gene). After lysing red blood cells, bone marrow cells were cultured at the cell density of 2 × 106/ml in RPMI 1640medium in 10cm dish (BD Biosciences, Durham, NC) at 37 °C, 5% CO2, supplemented with 10% fetal calf serum (FCS, cat# 100-106, Gemini Bio-Products, West Sacramento, CA), 2 mM L-glutamine (cat#G7513, Sigma, St. Louis, MO), 1X MEM non-essential amino acids (cat# 11140-050, Gibco, Grand island, NY), 1mM sodium pyruvate (cat#25-000-CI, Cellgro, Manassas, VA), 0.5mM beta-mercaptoethanol (cat#M3148, Sigma),100U/ml penicillin G, 100μg/ml streptomycin (cat#30-002-CI, Cellgro). For making neutrophils, the bone marrow cells were cultured in the growth medium that contains 100ng/ml recombinant G-CSF (cat# 250-05, PeproTech) alone. Every 3 days, the floating cells were harvested and re-cultured in fresh growth medium. On day 8, cells floating in the supernatants were subjected to flow cytometry analysis for Ly-6G or Gr1 positivity. When the positivity reached >80%, the cells were used as neutrophils in individual experiments.
2.3. Chemotaxis assay
The chemotaxis was measured in 24-well plates each well with a ThinCert™ Multiwell Plate Insert (cat#662631, Greiner Bio-One, Monroe, NC). The pore size of the insert bottom translucent membrane (PET) is 3μm. Neutrophils derived from bone marrow as described above were added into the insert or the upper chamber in 200μl chemotaxis medium (RPMI1604 with 1% sterile LPS-free BSA (Cat#A8806, Sigma, USA) at the cell density of 2 × 106 cells per ml. The chemoattractants were placed at concentrations as listed in individual experiments in the well below or the lower chamber filled with 800μl of the same chemotaxis medium. After two hours of incubation at 37°C, the cells in both upper and lower chambers were counted, based on which, the % of neutrophils migrated from the upper to lower chambers was calculated from 3 independent experiments.
2.4. Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to measure cytokines as described previously [7]. For measuring cytokines in culture supernatants, the endometrial epithelial cells or bone marrow-derived cells were grown in 24 well plates at the cell density of 1.5 × 105/ml for MS74 [an immortalized human vaginal epithelial cell line kindly provided by Dr. Robert Schenken from Department of Obstetrics and Gynecology, University of Texas Health Science Center at San Antonio; ref: [16]] or 1 × 106/ml for bone marrow-derived neutrophils. Cells were stimulated with LPS (Cat# tlrl-3pelps), Pgp3, LL-37 or Pgp3/LL-37 complex at different concentrations as indicated in individual experiments for 24h. The supernatants with or without dilutions were used for cytokine measurement. The LL-37/Pgp3 complex was formed by pre-incubating the two together in cell culture medium for 30min at 37°C. ELISA kits were used for measuring mouse IL-6 (Cat# 431301, Biolegend, San Diego, CA), mouse MIP-2 (Cat# DY452, R&D systems, Minneapolis, MN), human IL-6 (Cat# 555220) and human IL-8 (Cat# 555244, both from BD Bioscience, San Jose, CA) respectively.
2.5. Statistics
For quantitative data, Wilcoxon rank sum, while for qualitative data, Fisher exact test, were used.
Results
3.1. Cathelicidin peptide LL-37 is significantly induced in the genital tracts of women diagnosed positive for C. trachomatis
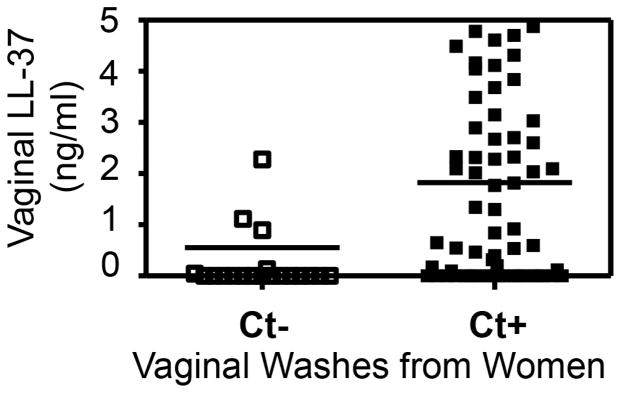
To provide direct evidence that C. trachomatis infection can induce LL-37 in women’s genital tracts, we took advantage of the remaining vaginal wash samples collected during previous studies [2, 17–19] for measuring LL-37 using an ELISA. These wash samples no longer have identifiable patient information. However, the chlamydial diagnosis results associated with these samples are available. During the previous studies, prior to the collection of vaginal wash with 50ml PBS from each woman, vaginal swabs were taken for diagnosing sexually transmitted infections including Chlamydia and Mycoplasma. Samples from 20 women diagnosed negative for any sexually transmitted infections and 67 diagnosed positive for C. trachomatis alone were used in the current experiment (Fig. 1). Since the samples were collected in large volume (already diluted), we used neat and a two-fold serial dilution of each of these samples for measuring LL-37. We found that women diagnosed positive for C. trachomatis produced significantly higher levels of LL-37 than those negative for C. trachomatis, suggesting that LL-37 were induced by C. trachomatis infection in the genital tracts of these women.
Fig. 1. The cathelicidin peptide LL-37 is induced in the genital tracts of women with C. trachomatis infection.
The remaining vaginal wash samples collected during previous studies were measured for LL-37 using ELISA. These wash samples had no identifiable patient information. Prior to the collection of vaginal wash with 50ml PBS from each woman, vaginal swabs were taken for diagnosing Chlamydia. Samples from 20 women diagnosed negative for C. trachomatis (Ct-, open bar) and 67 diagnosed positive (Ct+, solid bar) were used in the current experiment. Each sample was measured using neat solution in duplicate and the result was calculated into ng per ml of vaginal wash and displayed in mean plus/minus standard error from each group along the Y-axis. Note that women diagnosed positive for C. trachomatis produced significantly higher levels of LL-37 than those negative for C. trachomatis (**p<0.001, Wilcoxon Rank Sum).
3.2. LL-37 induction of inflammatory cytokines in human vaginal epithelial cells is inhibited by Pgp3
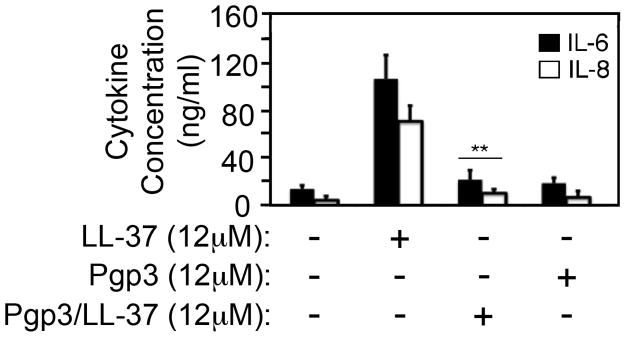
Having demonstrated that LL-37 is induced in women’s genital tracts by C. trachomatis, we evaluated whether LL-37 could induce cytokine production in human vaginal epithelial cell line MS74 and whether Pgp3 could modulate the LL-37 pro-inflammatory activity (Fig. 2). Besides its antimicrobial activity, LL-37 is known to also activate gingival fibroblast [20] and epithelial [21] cells to produce pro-inflammatory cytokines. We focused on IL-6 and IL-8 because both IL-6 [22] and IL-8 (MIP-2 in mouse) have been shown to play essential roles in chlamydial infection and pathogenesis [23]. We found that LL-37 at 12 μM induced both IL-6 and IL-8 in the supernatants of MS74 cultures while Pgp3 at the same molar concentration failed to do so. More importantly, Pgp3 completely blocked LL-37 induction of cytokine secretion by MS74 epithelial cells. Since Pgp3 is known to form stable complex with LL-37 [12], the above observation has demonstrated that Pgp3 can inhibit the proinflammatory activity of LL-37 by forming stable complexes between these two, which may help dampen the initial inflammatory responses that are detrimental to chlamydial invasion of the genital tract epithelial tissues.
Fig. 2. LL-37 induction of inflammatory cytokines in human endometrial epithelial cells is inhibited by chlamydial Pgp3.
The endometrial epithelial cell line MS74 was set up at 1.5 × 105 cells/ml/well in a 24 well plate and the cells were treated with or without 12μM of LL-37, Pgp3 or Pgp3/LL-37 complexes for 24h at 37°C. The supernatants were collected for measuring IL-6 (solid bar) and IL-8 (open) and the results were calculated from 3 independent experiments each with duplicates and displayed as ng/ml along the Y-axis (mean plus/minus standard error). Note that although LL-37 induced significant levels of IL-6 & IL-8, the LL-37 proinflammatory activity was blocked by forming complexes with Pgp3 (**p<0.01 (Wilcoxon ran sum). The Pgp3/LL-37 complexes were formed by pre-incubating the two together each at 12μM in cell culture medium for 30min at 37°C before applying the co-incubation mixtures to the cell culture wells.
3.3. LL-37 induction of neutrophil chemotaxis is blocked by Pgp3
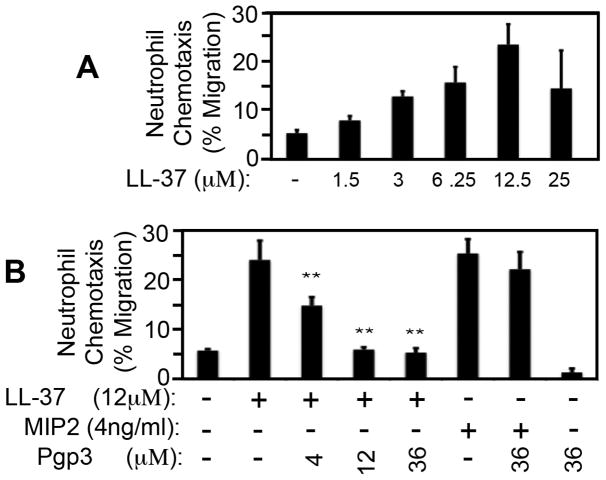
LL-37 can also function as a chemoattractant for inflammatory cells [13]. Neutrophils have been shown to play critical roles in both controlling chlamydial infection [24–26] and promoting chlamydial pathogenesis [26]. We evaluated whether Pgp3 can interfere with LL-37’s ability to recruit neutrophils (Fig. 3). We used bone marrow-derived neutrophils made from C3H/Hej mice as the responder cells for chemotaxis in order to minimize the impact of lipopolysaccharide (LPS, C3H/Hej mice lack the ability to respond to LPS due to a spontaneous mutation in toll-like receptor 4 gene or tlr4). We found that LL-37 induced neutrophil migration in a dose dependent manner (Fig. 3A). Neutrophils migrated from the upper to lower chambers in response to LL-37 placed in the lower chamber with a maximal neutrophil migration of ~25% when LL-37 was used at 12μM. We then used this concentration for the following Pgp3 inhibition experiment (Fig. 3B). We found that Pgp3 alone at 36μM did not induce neutrophil migration. However, administration of Pgp3 to LL-37-containing wells completely blocked LL-37 induction of neutrophil migration. The blockade effect increased with increasing amounts of Pgp3 ranging from 4μM (molar ratio of LL-37 to Pgp3=3:1) to 12μM (1:1). Once equal molar ratio is reached, the blockade effect plateaued. As a specificity control, Pgp3 failed to block neutrophil migration induced by the chemokine MIP-2 (IL-8), suggesting that the inhibitory effect of Pgp3 on LL-37 might be due to specific binding of Pgp3 to LL-37.
Fig. 3. LL-37 induction of neutrophil chemotaxis is blocked by Pgp3.
(A) Mouse bone marrow-derived neutrophils were used for measuring chemotaxis. The neutrophils were placed in upper chamber in 200μl chemotaxis medium at the cell density of 2 million cells per ml while the chemoattractant LL-37 in different concentrations as listed at the bottom of the figure was added to the lower chambers filled with 800μl of the same chemotaxis medium. The upper and lower chambers were separated by 3μM pore-size of inserts. After two hours of incubation at 37°C, the cells in both upper and lower chambers were counted, based on which, the % of neutrophils migrated from the upper to lower chambers was calculated from 3 independent experiments and displayed along the Y-axis. Note that LL-37 at 12μM exhibited strongest chemoattraction ability, which was used for testing whether Pgp3 could affect LL-37-induced chemoattraction in (B). The experiments in (B) were carried out as described above except that different chemoattractants were prepared, including LL-37 at 12μM alone, LL-37 pre-incubated with varying concentrations of Pgp3, MIP2 at 4ng/ml alone, MIP2 pre-incubated with 36μM Pgp3 and Pgp3 alone at 36μM. The pre-incubation was carried out by mixing LL-37 or MIP2 with Pgp3 at the concentrations as indicated at the bottom of the figure for 30min at 37°C in chemotaxis medium and 800μl of the mixtures was added to the corresponding lower chambers. Note that the % of neutrophils migrated into the lower chambers that contain the LL-37/Pgp3 mixtures were significantly lower than that from the well with LL-37 alone (**p<0.01, Wilcoxon ran sum).
3.4. Pgp3 induction of cytokine production in neutrophils is enhanced by LL-37
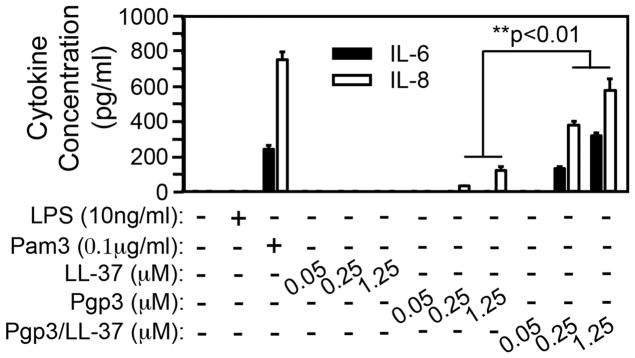
We have previously shown that Pgp3 is capable of activating mouse macrophages to produce inflammatory cytokines [7]. Since neutrophils are more relevant to chlamydial pathology [27] and C. muridarum-deficient in Pgp3 is attenuated in inducing pathology in the upper genital tract [28], we next tested whether Pgp3 can contribute to chlamydial pathogenesis by stimulating neutrophils to release inflammatory cytokines (Fig. 4). Since neutrophils were made from C3H/Hej mice, LPS was not able to activate these neutrophils to secrete cytokines while the TLR2 ligand Pam3 stimulated the same neutrophils to secrete both IL-6 and IL-8 as a positive control. We further found that Pgp3 but not LL-37 was able to stimulate neutrophils to secrete IL-8. Interestingly, the proinflammatory activity of Pgp3 was significantly enhanced by forming complexes with LL-37. The Pgp3/LL-37 complexes induced significant levels of both IL-6 and IL-8 at 0.25μM. The cytokine levels continued to rise as the concentrations of the complexes increased.
Fig. 4. Pgp3 induction of cytokine production in neutrophils is enhanced by forming complexes with LL-37.
Bone marrow-derived neutrophils made from C3H/HeJ mice (with TLR4 mutation, thus unable to respond to LPS stimulation) were plated 1 × 106 cells/ml/well in a 24 well plate. The cells were treated with or without 10ng/ml LPS (TLR4 ligand as a negative control), Pam3 (TLR2 ligand as positive control), LL-37 or Pgp3 alone or Pgp3/LL-37 complexes at 0.05, 0.25 and 1.25μM as indicated along the X-axis for 24h at 37°C. The supernatants were collected for measuring both IL-6 (solid bar) and IL-8 (open). The cytokine concentrations were calculated from 3 independent experiments and expressed as ng/ml as shown along the Y-axis. Note that Pgp3 but not LL-37 alone stimulated minimal levels of cytokines and the proinflammatory activity of Pgp3 was significantly enhanced by forming complexes with LL-37 (**p<0.01, Wilcoxon rank sum).
4. Discussion
Although the precise mechanisms of chlamydial pathogenicity remains unclear, the role of plasmid in chlamydial pathogenicity has recently been appreciated [29]. The strongest evidence came from the observations that plasmid-free [4, 5] or Pgp3-deficient [28] chlamydial organisms were highly attenuated in inducing pathology in the mouse upper genital tract or primate ocular tissues [6]. Among all the plasmid-encoded proteins [30], Pgp3 is the only protein that is secreted into host cell cytosol [7] and highly immunogenic [8, 11] during chlamydial infection. Pgp3 is the first chlamydial inhibitor of host antimicrobial peptides and it neutralizes the antichlamydial activity of the cathelicidin LL-37 peptide by binding to LL-37 with an extremely high affinity [12].
Besides its direct microbicidal effect, LL-37 is also known to amplify innate defense responses [20, 21, 31–33]. In the current study, we presented evidence that LL-37 was indeed induced in women’s genital tracts during C. trachomatis infection and it both activated human endometrial epithelial cells to secrete proinflammatory cytokines and recruited neutrophils. However, these proinflammatory activities of LL-37 were completely blocked by the chlamydial plasmid-encoded Pgp3 probably due to the stable complexes formed between LL-37 and Pgp3. Thus, besides its neutralization of the LL-37 antichlamydial activity, Pgp3 can also inhibit the LL-37’s proinflammatory activities, which may benefit chlamydial survival and ascending infection, especially, during the early stage of chlamydial infection.
We also found that Pgp3 by itself was able to activate neutrophils to secrete inflammatory cytokines, which is consistent with our previous observation that Pgp3 activated macrophages to secrete pro-inflammatory cytokines [7]. Interestingly, we further found that this pro-inflammatory activity of Pgp3 was enhanced by its interaction with LL-37. The Pgp3/LL-37 interaction, resulting in the inhibition of LL-37’s proinflammatory activities but the enhancement of the Pgp3-induced inflammation, may allow Chlamydia to be in the driver seat for manipulating host inflammatory responses to both promote chlamydial survival in the infected hosts and spreading to new hosts. Although inflammation at the early stage of chlamydial infection may be detrimental to chlamydial survival/ascension, it has been hypothesized that maintaining certain level of inflammation may promote chlamydial spreading between different hosts [14]. An unintended consequence of the prolonged inflammation induced by the stable Pgp3/LL-37 complex may be the development of long-term pathology in the upper genital tract.
The pathological basis of Chlamydia-induced sequelae in the female genital tract is long-lasting/irreversible fibrosis. Not all tubal inflammatory responses induced by chlamydial organisms can lead to long-lasting fibrosis [34]. Thus, defining the properties of tubal inflammation that leads to long-lasting tubal fibrosis has been a hot topic. The current study has shown that the Pgp3/LL-37 complex prevented LL-37 induction of IL-6/8 in epithelial cells, thus delaying the onset of inflammation. However, the same complex promoted the production of the inflammatory cytokines in neutrophils, thus sustaining inflammation once neutrophils arrive at the site of infection. IL-8 is a neutrophil chemoattractant factor that can also promote survival of neutrophils [35]. The Pgp3/LL-37 complex may promote autocrine signaling between IL-8 and neutrophils, which may be self-sustainable. Neutrophil longevity has been shown to significantly contribute to chlamydial induction of long-lasting tubal pathology [27, 36]. IL-6 has also been shown to play a critical role in chlamydial induction of long-lasting hydrosalpinx [22]. These analyses suggest that the stable Pgp3/LL-37 complex trigger and maintain an autocrine loop between inflammatory cytokines and long-lived neutrophils, leading to irreversible tubal fibrosis. It will be interesting to test whether the Pgp3/LL-37 complex is indeed necessary and/or sufficient for chlamydial induction of long-lasting tubal fibrosis/hydrosalpinx.
Acknowledgments
This work was supported in part by grants from Natural Science Foundation of China (NO:31770194 to S. Hou) and US National Institute of Health (R01AI121989 to G. Zhong).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention C; USDoHaH Services, editor. Sexually Transmitted Disease Surveillance. Atlanta, GA: 2016. https://www.cdc.gov/std/stats16/default.htm. 2017. [Google Scholar]
- 2.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, et al. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol. 2012;119:1009–16. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas NS, Lusher M, Storey CC, Clarke IN. Plasmid diversity in Chlamydia. Microbiology. 1997;143:1847–54. doi: 10.1099/00221287-143-6-1847. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell CM, Ingalls RR, Andrews CW, Jr, Scurlock AM, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179:4027–34. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 5.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, et al. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun. 2014;82:983–92. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kari L, Whitmire WM, Olivares-Zavaleta N, Goheen MM, Taylor LD, Carlson JH, et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J Exp Med. 2011;208:2217–23. doi: 10.1084/jem.20111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76:3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Zhong Y, Lei L, Wu Y, Wang S, Zhong G. Antibodies from women urogenitally infected with C. trachomatis predominantly recognized the plasmid protein pgp3 in a conformation-dependent manner. BMC Microbiol. 2008;8:90. doi: 10.1186/1471-2180-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J Bacteriol. 2010;192:6017–24. doi: 10.1128/JB.00847-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galaleldeen A, Taylor AB, Chen D, Schuermann JP, Holloway SP, Hou S, et al. Structure of the Chlamydia trachomatis immunodominant antigen Pgp3. J Biol Chem. 2013;288:22068–79. doi: 10.1074/jbc.M113.475012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J Immunol. 2010;185:1670–80. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- 12.Hou S, Dong X, Yang Z, Li Z, Liu Q, Zhong G. Chlamydial plasmid-encoded virulence factor Pgp3 neutralizes the antichlamydial activity of human cathelicidin LL-37. Infect Immun. 2015;83:4701–9. doi: 10.1128/IAI.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191:4895–901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11:44–51. doi: 10.1016/s0966-842x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 15.Comanducci M, Ricci S, Cevenini R, Ratti G. Diversity of the Chlamydia trachomatis common plasmid in biovars with different pathogenicity. Plasmid. 1990;23:149–54. doi: 10.1016/0147-619x(90)90034-a. [DOI] [PubMed] [Google Scholar]
- 16.Greene W, Xiao Y, Huang Y, McClarty G, Zhong G. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect Immun. 2004;72:451–60. doi: 10.1128/IAI.72.1.451-460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaylock MW, Musatovova O, Baseman JG, Baseman JB. Determination of infectious load of Mycoplasma genitalium in clinical samples of human vaginal cells. J Clin Microbiol. 2004;42:746–52. doi: 10.1128/JCM.42.2.746-752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korte JE, Baseman JB, Cagle MP, Herrera C, Piper JM, Holden AE, et al. Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am J Reprod Immunol. 2006;55:265–75. doi: 10.1111/j.1600-0897.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 19.Thurman AR, Musatovova O, Perdue S, Shain RN, Baseman JG, Baseman JB. Mycoplasma genitalium symptoms, concordance and treatment in high-risk sexual dyads. Int J STD AIDS. 2010;21:177–83. doi: 10.1258/ijsa.2009.008485. [DOI] [PubMed] [Google Scholar]
- 20.Montreekachon P, Chotjumlong P, Bolscher JG, Nazmi K, Reutrakul V, Krisanaprakornkit S. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011;46:327–37. doi: 10.1111/j.1600-0765.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- 21.Montreekachon P, Nongparn S, Sastraruji T, Khongkhunthian S, Chruewkamlow N, Kasinrerk W, et al. Favorable interleukin-8 induction in human gingival epithelial cells by the antimicrobial peptide LL-37. Asian Pac J Allergy Immunol. 2014;32:251–60. doi: 10.12932/AP0404.32.3.2014. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Tian Q, Wang L, Xue M, Zhong G. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect / Institut Pasteur. 2017;19:536–45. doi: 10.1016/j.micinf.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen SJ, Eckmann L, Quayle AJ, Shen L, Zhang YX, Anderson DJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naglak EK, Morrison SG, Morrison RP. Neutrophils are central to antibody-mediated protection against genital Chlamydia. Infect Immun. 2017:85. doi: 10.1128/IAI.00409-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barteneva N, Theodor I, Peterson EM, de la Maza LM. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–3. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacy HM, Bowlin AK, Hennings L, Scurlock AM, Nagarajan UM, Rank RG. Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections. Infect Immun. 2011;79:1889–97. doi: 10.1128/IAI.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazer LC, O’Connell CM, Andrews CW, Jr, Zurenski MA, Darville T. Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect Immun. 2011;79:4029–41. doi: 10.1128/IAI.05535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, et al. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun. 2014;82:5327–35. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong G. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol. 2017;25:141–52. doi: 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson JH, Whitmire WM, Crane DD, Wicke L, Virtaneva K, Sturdevant DE, et al. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–83. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi KY, Chow LN, Mookherjee N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun. 2012;4:361–70. doi: 10.1159/000336630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byfield FJ, Kowalski M, Cruz K, Leszczynska K, Namiot A, Savage PB, et al. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J Immunol. 2011;187:6402–9. doi: 10.4049/jimmunol.1102185. [DOI] [PubMed] [Google Scholar]
- 33.Byfield FJ, Wen Q, Leszczynska K, Kulakowska A, Namiot Z, Janmey PA, et al. Cathelicidin LL-37 peptide regulates endothelial cell stiffness and endothelial barrier permeability. Am J Physiol Cell Physiol. 2011;300:C105–12. doi: 10.1152/ajpcell.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, et al. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PloS one. 2014;9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int. 1998;53:84–91. doi: 10.1046/j.1523-1755.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, et al. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun. 2014;82:2688–96. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]