Abstract
Introduction:
OnabotulinumtoxinA (OnabotA) is a well-described treatment for Neurogenic Overactive Bladder (NOAB) and while its motor effects on detrusor muscle is extensively studied, its sensory effects are not. The aim of this study was to evaluate the impact of intradetrusor OnabotA injection on brain activity in female multiple sclerosis (MS) patients with NOAB.
Methods:
We conducted a prospective study of 12 women with stable MS and NOAB using concurrent functional magnetic resonance imaging (fMRI) and Urodynamic Studies (UDS) prior to and 6 to 10 weeks following OnabotA. Individual fMRI activation maps at the time of strong urgency were averaged prior and post OnabotA where areas of significant activation were identified.
Results:
fMRI activation increased post OnabotA in the right cingulate body (p=0.0012), left posterior cingulate (p=0.02), left anterior cingulate (p=0.0015), right prefrontal cortex (p=0.0015), insula (p=0.0138) and pons micturition center (p=0.05). Areas that showed decreased activity were sparse and included the left cerebellum (p=0.001), left fusiform gyrus (p=0.065) and bilateral lentiform nucleus (p=0.026).
Conclusions:
Intradetrusor injection of OnabotA appears to increase the activity most of the brain regions known to be involved in the sensation and process of urinary urgency in female MS patients with NOAB. This is the first study of its kind to evaluate the possible effects of OnabotA at the human brain level where sensory awareness is located. This pattern of activation may be used to phenotype patients further to optimize therapy or to uncover sensory effects of OnabotA beyond the bladder.
Keywords: Botox, Bladder, functional MRI, overactive bladder
Introduction
Multiple Sclerosis (MS) is a chronic multifocal demyelinating disease that affects the central nervous system, mainly the brain. Urinary frequency, urgency, or urge urinary incontinence are referred to as neurogenic overactive bladder (NOAB) and are the most common symptoms, occurring in 37% to 99% of MS patients.1 Both urgency and urge urinary incontinence impact health-related quality of life negatively.2
OnabotulinumtoxinA (OnabotA) blocks the release of acetylcholine at the neuromuscular junction and leads to a temporary chemodenervation of the bladder. Motor effects of OnabotA on the bladder have been extensively studied,3 which led to its approval by the United States Food and Drug Administration in August of 2011 for the treatment of NOAB in patients with MS.4-6
Despite significant amounts of animal and human research on the motor effects of OnabotA, its sensory effects specifically correlating to brain regions for perception/localization of bladder fullness, urgency, and frequency in humans is not well-understood.7 With advances in neuroimaging, functional Magnetic Resonance Imaging (fMRI) has been used to evaluate brain responses to cold sensation8 and urinary urgency9, elucidating supraspinal areas involved in the perception of bladder sensation. Whether or not intradetrusor OnabotA effects in humans extend to the brain level remains to be elucidated. fMRI potentially represents an unprecedented modality to determine the effects of OnabotA on regions of the brain regulating micturition.
The aim of this study was to evaluate the effects of intradetrusor OnabotA on brain activity in female MS patients with NOAB using concurrent fMRI and Urodynamic Study (UDS) in a priori Regions of Interest (ROI) at the time of strong desire to void (Full Urge). We hypothesized that intradetrusor OnabotA in our cohort modulates (decreases) the activity in ROIs as characterized by fMRI Blood Oxygen Level Dependent (BOLD) activation patterns.
Method
We performed an observational prospective study approved by our Institutional Review Board prior to patient selection and registered at ClinicalTrials.gov (Identifier: NCT03033355). Complete and detailed protocol for this study is published and available for referrence.10
Patient selection and recruitment
Inclusion criteria consisted of adult female patients with clinically stable MS for ≥3 months before screening and an Expanded Disability Status Score (EDSS) ≤6.5 with urinary frequency or urgency who were refractory to behavioral therapies and oral pharmacological management for NOAB and were interested in OnabotA intradetrusor injection. Men were excluded from the study to avoid confounding by prostate and bladder outlet pathology. Other exclusion criteria include severe debilitating disease, pregnancy or planning to become pregnant, nursing, MRI contraindications, history of augmentation cystoplasty, and presence of other neurological diseases. Patients are screened and recruited from our Neurourology Clinic.
Evaluation
Each patient underwent a detailed history and physical examination and completed a baseline clinic UDS. The following questionnaires and assessments were obtained at baseline and six to ten weeks post OnabotA injection: Urogenital Distress Inventory (UDI-6) and Incontinence Impact Questionnaire (IIQ-7), MRI Safety Screening Questionnaire and Hamilton Anxiety Rating Scale, Post-voidresidual (PVR) volume and urinalysis. Patients were seen for follow-up in the clinic at 2 and 6 weeks following OnabotA treatment for symptom evaluation, urinalysis and measurement of PVR volume. If there were clinical signs of increased PVR volume or urinary retention, clean intermittent catheterizations (CIC) was initiated.
Intradetrusor OnabotA injection
One hundred (in two of the spontaneous voiders) and 200 (in CIC dependent patients) units of OnabotA was injected cystoscopically throughout the bladder, including the trigone.
Simultaneous urodynamic testing and fMRI
We used our previously established fMRI/UDS platform in healthy females and MS patients.11, 12 Double lumen 7 Fr MRI-compatible catheters were placed in the bladder and rectum. A Phillips Ingenia 3.0T full body MRI with a standard 12 channel head coil was used. Instructions to communicate using right hand signals representing ‘strong desire to void (full urge)’, ‘voiding’ and ‘voiding completed’ were given to the patient before imaging. To avoid auditory or other extensive brain stimulation not related to the voiding process, simple signs were shown to the patient when filling of the bladder is begun and when filling is stopped. Also, in order to keep our noise-to-signal ratio low, all stimulators including any extra visual stimuli and the urodynamic machine were removed from the MRI room. Total scan times were limited to 45 min. After acquisition of a high-resolution three-dimensional (3D) brain scan for anatomical reference (T1-weighted, isotropic 3D resolution of 0.7 mm), echo-planar imaging (EPI; repetition time 3 s, in-plane resolution 3.4 mm, slice thickness 4 mm) during two functional tasks are performed to record the Blood Oxygen Level Dependent (BOLD) signal.
First, instruction signals from the patient’s right hand (tasks) were performed without actual voiding and scanned separately to obtain a baseline for fMRI activation caused from signaling alone. Afterwards, urodynamic testing and the filling task are simultaneously initiated. The bladder is filled at 50 mL/min with room temperature, sterile, normal saline solution until the participant indicates a strong desire to void. Filling is stopped and participants will be asked to hold the urine for 30 s. Afterwards, patients will be allowed to void or attempt to void into absorbent pads on the scanner table. Bladder was aspirated if patient was unable to void. To create a block design fMRI task, this sequence was repeated up to four times. Final Catheterizable PVR volume was recorded. This procedure was performed before treatment and 6–10 weeks following OnabotA.
Data analysis and statistics
Analysis of the BOLD fMRI images was performed as described previously11, 12. A healthy female cohort described in a previous report was used as healthy controls (HC)12. AFNI software (NIMH, https://afni.nimh.nih.gov/afni/) was used for the fMRI BOLD analysis. Structural and functional images were registered to each other. The EPI fMRIs were motion-corrected. Patients with large motion (>4.5 mm) were excluded from analysis. Voxel activation was identified at the time of ‘strong desire to void (full urge)’ and BOLD fMRI activation maps were calculated using standard analysis with the generalized linear model (GLM). Group analysis was performed by transforming data into the Talairach space, and significantly activated voxels were identified using Student’s t-test. Comparisons were drawn between participants with MS comparing baseline scans to the post-OnabotA treatment. In addition, MS patients were compared pre and post-OnabotA to the HC cohort. We determined that 12 subjects would provide 80% power at the single voxel level for typical activation accounting for intra-and inter-subject variability, based on prior fMRI power calculation data.13 In most trials using fMRI evaluating lower urinary tract function (specifically the two most recent and similar trials), power is set at 12 patients14, 15 Based on prior functional neuroimaging studies evaluating areas related to bladder fullness in nonneurogenic overactive bladder women16, our analysis was focused on the following ROIs: cingulate cortex, insula, prefrontal cortex, pons, cerebellum, thalamus, and amygdala.
Results
Twelve female patient with MS and mean age of 43.9 (range of 38-71) and mean MS duration of 14.9 (range of 2-38) completed the study. Ten patients were ambulatory and two were wheelchair bound. All patients had lesions of varying sizes and location in their cerebrum. Table 1 summarizes the demographics and evaluations of the patients at baseline and following intradetrusor injection of OnabotA. Ten patients received 200 units of OnabotulinumtoxinA and two of the four patients who were spontaneous voiders received 100 units. Mean repeat OnabotA injection for our cohort was: 2.08 (0-7). Eight patients at baseline performed CIC and three out of four patients who were voiding spontaneously needed to initiate self-catheterization following treatment, therefore leaving eleven patients on CIC post OnabotA. All patients had an improvement in their total IIQ-7 and UDI-6 score (specifically Questions 1 and 2 which address the urinary frequency and urge urinary incontinence).
Table 1.
Patient demographics at baseline and following OnabotA injection. UUI: Urge Urinary Incontinence; CIC: Clean Intermittent Catheterization; UDI-6: Urogenital Distress Inventory; Q: question; IIQ-7: Incontinence Impact Questionnaire; UDS: Urodynamic Study; PVR: Post void residual; NDO: Neurogenic Detrusor Overactivity; MCC: Maximum Cystometric Capacity.
| MS Patients (mean age of 43.9) | Pre-OnabotA | Post-OnabotA |
|---|---|---|
| Voiding diary | ||
| UUI/day | 1.64 (0-4) | 0.42 (0-1) |
| CIC | 8 (66.7%) | 11 (91.7%) |
| Questionnaires | ||
| UDI-6 | 12.09 (6-21) | 6.27 (0-15) |
| UDI-6, Q1 (frequency) | 3.27 (2-4) | 1.55 (0-4) |
| UDI-6, Q2 (UUI) | 2.64 (0-4) | 1.1 (0-4) |
| IIQ-7 | 9.64 (0-18) | 4.36 (0-15) |
| UDS parameters | ||
| Final PVR (cc) | 116.2 (0-350) | 377.9 (120-600) |
| NDO during fMRI/UDS | 6 (50%) | 1 (8.3%) |
| MCC @1st fMRI fill (cc) | 134.5 (14-251) | 195.5 (51-401) |
Statistically significant increase in BOLD activation signals post OnabotA were observed in the right and left cingulate, the right prefrontal cortex, the insula, and the pons micturition center. Areas that showed decreased activity were sparse and included the left cerebellum (p=0.001), the left fusiform gyrus (p=0.065) and the bilateral lentiform nucleus (p=0.026), table 2.
Table 2.
Summary of fMRI activation patterns at the time of strong desire to void (Full Urge) in our a priori ROI coordinates using MNI space and RAS coordinates system, and the p value for each ROI (subtraction of post-pre OnabotA).
| ROI | R | A | S | t-value change | p value |
|---|---|---|---|---|---|
| Increased BOLD signal activity following OnabotA treatment | |||||
| Right Cingulate Body | 17 | 3 | 39 | 2.6 | 0.0012 |
| Left Posterior Cingulate | −7 | −37 | 22 | 2.6 | 0.02 |
| Left Anterior Cingulate | −3 | 24 | 8 | 3.5 | 0.0015 |
| Right Prefrontal Cortex | 3 | 51 | 46 | 2.6 | 0.0015 |
| Left Insula | −42 | 5 | 14 | 2.6 | 0.0138 |
| Pons Micturition Center | 4 | −30 | −22 | 2.4 | 0.05 |
| Decreased BOLD signal activity following OnabotA treatment | |||||
| Left Cerebellum | −33 | −64 | −51 | −3.9 | 0.001 |
| Left Fusiform Gyrus | −40 | 3 | −22 | −3.1 | 0.065 |
| Left Lentiform Nucleus | −17 | 3 | −9 | −2.1 | 0.026 |
| Right Lentiform Nucleus | 16 | 5 | −9 | −2.6 | 0.026 |
| Left Amygdala/parahippocampal Gyrus | −26 | −8 | −12 | −2.0 | 0.015 |
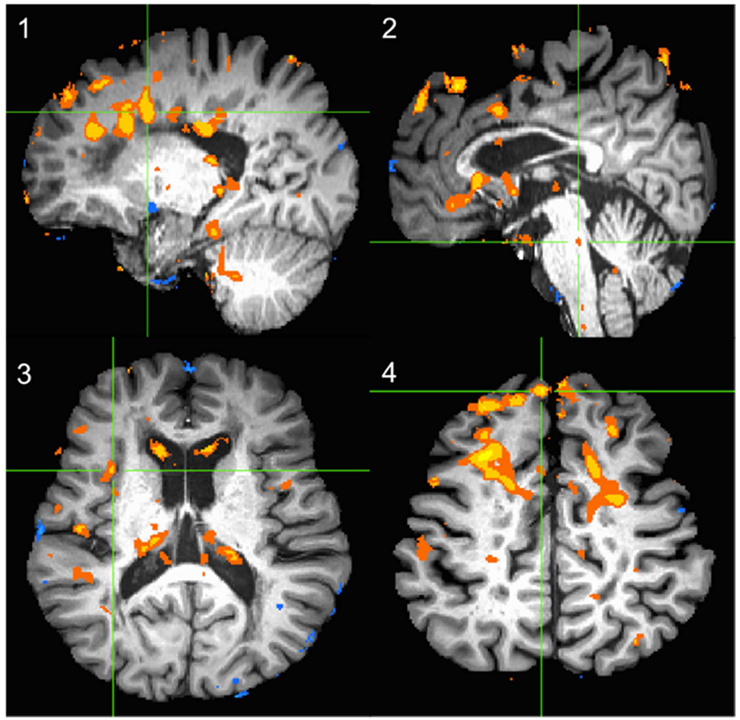
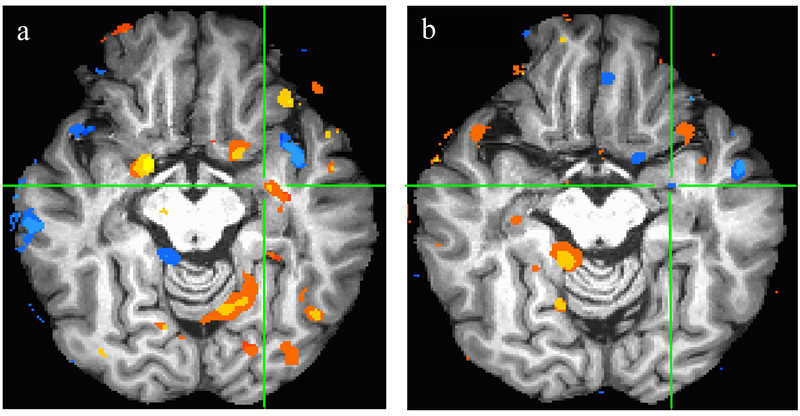
Figure 1 shows subtraction of post-pre OnabotA BOLD patterns at ROI overall. Figure 2 exemplary demonstrates the BOLD signal patterns in amygdala and parahippocampal gyrus at baseline and their decrease following OnabotA injection.
Figure 1.
The subtraction BOLD activation maps at the time of strong desire to void (Full Urge) of post-pre OnabotA for the ROIs. The green crosshair indicated the ROI in each panel. 1) Right Cingulate 2) Pons 3) Insula 4) Right Prefrontal Cortex
Figure 2.
Demonstrates Amygdala/parahippocampal gyrus at the time of strong desire to void (Full Urge) at baseline(a) with elevated blood flow and following OnabotA treatment (b) with decreased blood flow.
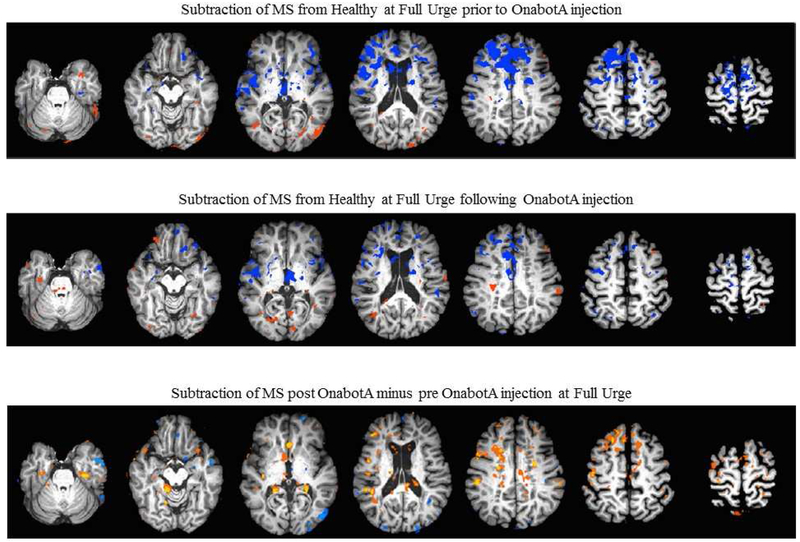
Following OnabotA injection, overall BOLD patterns in the brain showed less differences to the pattern of the HC cohort, with most changes being evident in the frontal lobes (figure 3).
Figure 3.
The subtraction of BOLD activation maps at the time of strong desire to void (Full Urge) of MS patients from Healthy Controls prior (top row) and following (middle row) OnabotA injection. The bottom row depicts the subtraction of BOLD activation maps of MS patients post OnabotA from pre OnabotA at the time of Full Urge.
Discussion
Studies suggest OnabotA in the bladder has several mechanisms of action besides its inhibitory effect on neurotransmitter release from parasympathetic nerve terminals on the detrusor muscle.7 In humans with overactive bladder, activation of specific receptors involved in afferent signaling pathways such as P2X2 and P2X3 are reduced by OnabotA, suggesting that OnabotA works by inhibiting both sensory and motor aspects of detrusor function.17 Moreover, some studies support the idea of a potential role of sensory purinergic pathways in the development of bladder overactivity and suggest that OnabotA may reduce purinergic transmission.18
Prior neuroimaging studies propose that specific regions of brain that are involved with processing sensation of bladder fullness and urgency in nonneurogenic overactive bladder to be cingulate, insular, and frontal (prefrontal) cortices and have increased activation patterns as the bladder volume increases.19 These areas appear to change following nonneurogenic OAB therapeutic interventions such as biofeedback20, anticholinergic medications21, and sacral neuromodulation14; displaying brain neuroplasticity.
Our trial is the first study of its kind to evaluate the possible effects of intradetrusor injection of OnabotA at the level of human brain where the perception and processing of fullness is located. Our results are complementary and similar in parts to the current literature, yet different in some aspects. We were able to show that injection of OnabotA in the bladder appears to have distinct effects on the activation pattern of brain regions associated with the sensation of bladder filling, urgency, and micturition. For the most part our results show increased activity post OnabotA injection (in cingulate and prefrontal cortices, insula, pons, and thalamus) which is in contrast to some of the studies where other interventions (biofeedback20, anticholinergic medications21, and sacral neuromodulation14, 15) for OAB were used. These differences could be due to multiple factors. Firstly, our cohort consists of MS patients with refractory neurogenic OAB which is inherently unlike and very unique by definition compared to idiopathic OAB patients in the abovementioned studies. Griffiths and colleagues showed that older community dwelling women with urge urinary incontinence who did not respond to biofeedback assisted pelvic floor physical therapy displayed a distinct neuroimaging brain activation pattern with medial prefrontal cortex being deactivated at baseline. In our cohort of MS patients with NOAB at baseline and prior to OnabotA injection, we observed a similar decreased BOLD activation (especially in left superior frontal gyrus) which could possibly be due to higher severity of NOAB symptoms experienced by our patients (Figure 3).
Our patients appear to be a different cohort of patients all together since these patients were already refractory to pelvic floor physical therapy and oral medications. Furthermore, due to the diffuse, multifocal involvement of brain plaques in patients with MS, symptom severity and impact on quality of life may vary from patient to patient.22, 23 MS lesions also affect functional connectivity between ROI at baseline and following NOAB management, making interpretation of these results interesting, yet more challenging. Despite this, following OnabotA injection overall BOLD signal patterns in the brain becomes less different between MS and HC at the time of full urge (figure 3).
Another finding in our cohort is that the entire micturition cycle, including the voiding phase changed following intradetrusor injection of OnabotA. Patients had an increase in the maximum cystometric capacity and post void residuals requiring de novo CIC in three out of four patients who were voiding spontaneously at baseline.24, 25 Such strong impact on the voiding phase (and specifically a negative effect) is in contrast to other therapies used in OAB. We believe this negative effect on the voiding phase of the micturition cycle could possibly have an effect on the brain; causing increased BOLD signal activation pattern in order to allow for voiding (or attempt of it).
Additional thought-provoking argument for our findings is that we are evaluating brain activation patterns post-OnabotA at much higher bladder volumes (larger MCCs). BOLD activation patterns from visceral organs (bowel and bladder 19, 26-28) have direct relationship with their capacity (distention related). Increased BOLD signals are seen at higher bladder and bowel volumes.. Increased signals displayed in our cohort post-OnabotA treatment could potentially be due to the fact that full urge is registered at a larger bladder volume and the data analysis is performed at that point.
Finally, we would like to discuss our intriguing findings in the amygdala and parahippocampal region. Amygdala is part of the limbic system and is responsible for processing negative and fearful sensations (such as urgency and urge urinary incontinence).27 The amygdala/parahippocampal region appears to demonstrate decreased BOLD signal activation after successful treatment of UUI by chronic neuromodulation29which is similar to our results where BOLD activation decreased following successful treatment of NOAB with OnabotA injection.
Further studies are undoubtedly needed in a larger population of patients (OAB and NOAB) undergoing OnabotA injection to increase our understanding of brain and potentially sensory effects of intradetrusor OnabotA.
Conclusion
This is the first study of its kind to evaluate the possible effects of intradetrusor injection of OnabotA at the human brain level where sensory awareness is located. BOLD activation patterns appear to increase in female MS patients with NOAB in most of the brain regions involved in sensation and processing of urinary urgency possibly due to data analysis being performed at higher bladder volumes. These effects are reversed in amygdala and parahippocampal region where the fear and anxiety (possibly due to urgency and UUI) is processed. This pattern of BOLD signal activation/deactivation pattern may be used to phenotype patients further to optimize therapy or to uncover sensory effects of OnabotA beyond the bladder.
Acknowledgements:
SUFU Research Foundation Chemodenervation Grant and K12 DK0083014 (RK is partially supported by the Multidisciplinary K12 Urologic Research Career Development Program from NIDDK)
Glossary
- BOLD
Blood Oxygen Level Dependent
- CIC
clean intermittent catheterization
- fMRI
functional Magnetic Resonance Imaging
- HC
Healthy Control
- OAB
Overactive Bladder
- OnabotA
OnabotulinumtoxinA
- MS
Multiple Sclerosis
- NOAB
neurogenic overactive bladder
- UDS
Urodynamic Study
Footnotes
Publisher's Disclaimer: DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Dillon BE, Lemack GE: Urodynamics in the evaluation of the patient with multiple sclerosis: when are they helpful and how do we use them? Urol Clin North Am, 41: 439, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Khalaf KM, Coyne KS, Globe DR et al. : The impact of lower urinary tract symptoms on health-related quality of life among patients with multiple sclerosis. Neurourol Urodyn, 35: 48, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Cruz F: Targets for botulinum toxin in the lower urinary tract. Neurourol Urodyn, 33: 31, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg D, Gousse A, Keppenne V et al. : Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol, 187: 2131, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cruz F, Herschorn S, Aliotta P et al. : Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol, 60: 742, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Kennelly M, Dmochowski R, Ethans K et al. : Long-term Efficacy and Safety of OnabotulinumtoxinA in Patients With Urinary Incontinence Due to Neurogenic Detrusor Overactivity: An Interim Analysis. Urology, 81: 491, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Jiang YH, Liao CH, Kuo HC: Current and potential urological applications of botulinum toxin A. Nat Rev Urol, 12: 519, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Mehnert U, Michels L, Zempleni MZ et al. : The Supraspinal Neural Correlate of Bladder Cold Sensation-An fMRI Study. Human Brain Mapping, 32: 835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhtz-Buschbeck JP, van der Horst C, Pott C et al. : Cortical representation of the urge to void: A functional magnetic resonance imaging study. Journal of Urology, 174: 1477, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Elizondo RA, Karmonik C, Boone TB et al. : Protocol for a prospective observational study of cortical lower urinary tract control changes following intradetrusor injection of botulinum toxin-A in patients with multiple sclerosis. BMJ Open, 7: e013225, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khavari R, Karmonik C, Shy M et al. : Functional Magnetic Resonance Imaging with Concurrent Urodynamic Testing Identifies Brain Structures Involved in Micturition Cycle in Patients with Multiple Sclerosis. J Urol, 197: 438, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shy M, Fung S, Boone TB et al. : Functional magnetic resonance imaging during urodynamic testing identifies brain structures initiating micturition. J Urol, 192: 1149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmond JE, Glover GH: Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods, 118: 115, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Weissbart SJ, Bhavsar R, Rao H et al. : Specific Changes in Brain Activity during Urgency in Women with Overactive Bladder after Successful Sacral Neuromodulation: A Functional Magnetic Resonance Imaging Study. J Urol, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Gill BC, Pizarro-Berdichevsky J, Bhattacharyya PK et al. : Real-Time Changes in Brain Activity during Sacral Neuromodulation for Overactive Bladder. J Urol, 198: 1379, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D: Neural control of micturition in humans: a working model. Nat Rev Urol, 12: 695, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Apostolidis A, Dasgupta P, Fowler CJ: Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol, 49: 644, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Apostolidis A, Jacques TS, Freeman A et al. : Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol, 53:1245, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Griffiths D, Derbyshire S, Stenger A et al. : Brain control of normal and overactive bladder. J Urol, 174: 1862, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Griffiths D, Clarkson B, Tadic SD et al. : Brain Mechanisms Underlying Urge Incontinence and its Response to Pelvic Floor Muscle Training. J Urol, 194: 708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontari MA, Mohamed FB, Lebovitch S et al. : Central nervous system findings on functional magnetic resonance imaging in patients before and after treatment with anticholinergic medication. J Urol, 183: 1899, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Kincses ZT, Ropele S, Jenkinson M et al. : Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler, 17: 681, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Charil A, Zijdenbos AP, Taylor J et al. : Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. Neuroimage, 19: 532, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Rovner E, Dmochowski R, Chapple C et al. : OnabotulinumtoxinA improves urodynamic outcomes in patients with neurogenic detrusor overactivity. Neurourol Urodyn, 32: 1109, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Kalsi V, Gonzales G, Popat R et al. : Botulinum injections for the treatment of bladder symptoms of multiple sclerosis. Ann Neurol, 62: 452, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kavia R, Dasgupta R, Critchley H et al. : A functional magnetic resonance imaging study of the effect of sacral neuromodulation on brain responses in women with Fowler’s syndrome. BJU Int, 105: 366, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Griffiths D, Tadic SD: Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn, 27: 466, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Berman SM, Naliboff BD, Suyenobu B et al. : Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci, 28: 349, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blok BF, Groen J, Bosch JL et al. : Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int, 98: 1238,2006 [DOI] [PubMed] [Google Scholar]