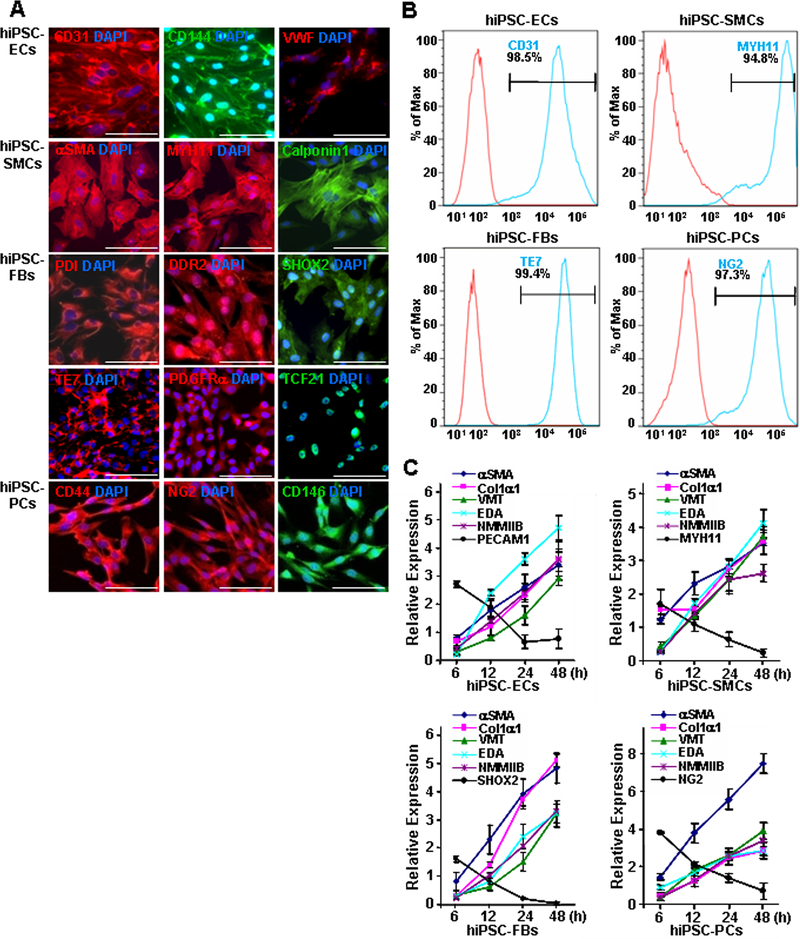
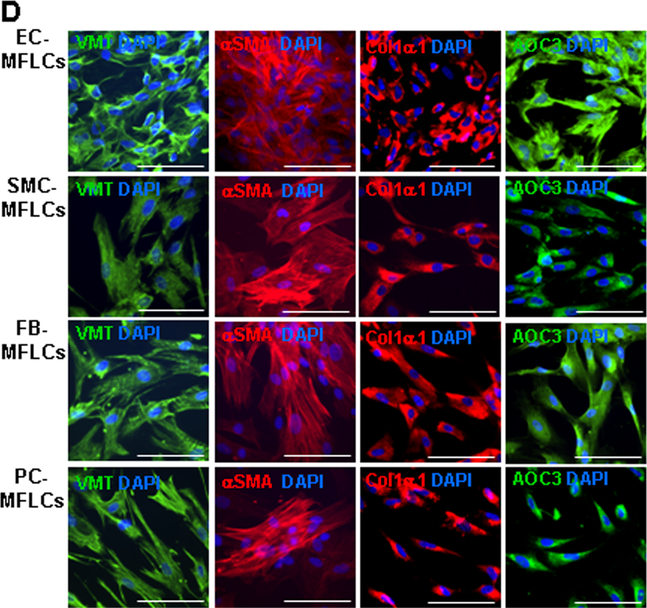
Figure 1. hiPSC-derived non-cardiomyocyte cardiac cells (hiPSC-NMCCs) were differentiated into myofibroblast-like cells (MFLCs).
(A) hiPSCs were differentiated into endothelial cells (ECs), smooth-muscle cells (SMCs), fibroblasts (FBs), and pericytes (PCs); then, the lineages of the differentiated cells were confirmed via immunofluorescence analysis of CD31, CD144, and VWF in hiPSC-ECs; αSMA, MYH11, and calponin1 in hiPSC-SMCs; PDI, DDR2, SHOX2, TE7, PDGFRα, and TCF21 in hiPSC-fibroblasts; and CD44, NG2, and CD146 in hiPSC-pericytes. Nuclei were counterstained with DAPI (Bar=100 μm). (B) The purity of the hiPSC-derived NMCCs was determined via flow-cytometry analysis of CD31 (blue) expression for hiPSC-ECs, MYH11 expression (blue) for hiPSC-SMCs, TE7 expression (blue) for hiPSC-fibroblasts, and NG2 expression (blue) for hiPSC-pericytes. Control assessments (red) were performed with non-specific antibodies of the same class and type. (C) hiPSC-NMCCs were differentiated into MFLCs over a 48-hour period, and mRNA levels of the myofibroblast markers αSMA, Col1α1, VMT, NMMIIB, and EDA and of markers for the cells’ lineages of origin (ECs: PECAM1; SMCs MYH11; fibroblasts: SHOX2; pericytes: NG2) were measured at the indicated time points via quantitative RT-PCR. (D) MFLCs differentiated from hiPSC-ECs (EC-MFLCs), -SMCs (SMC-MFLCs), -fibroblasts (FB-MFLCs), and pericytes (PC-MFLCs) were immunofluorescently stained for expression of the myofibroblast markers VMT, αSMA, Col1α1, and AOC3; nuclei were counterstained with DAPI (bar=100 μm). VWF: von Willebrand factor, αSMA: α smooth-muscle actin, MYH11: smooth muscle myosin heavy chain 11, PDI: protein disulfide isomerase, DDR2: discoidin domain receptor 2, SHOX2: short-stature homeobox 2, TE7: fibroblast marker clone TE7, PDGFRα: platelet-derived growth factor receptor alpha, TCF21: transcription factor 21, NG2: neural/glial antigen 2, Col1α1: collagen 1α1, VMT: vimentin, NMMIIB: non-muscle myosin IIB, EDA: fibronectin extra domain A, PECAM1 (CD31): platelet and endothelial cell adhesion molecule 1, AOC3: amine oxidase copper-containing 3.