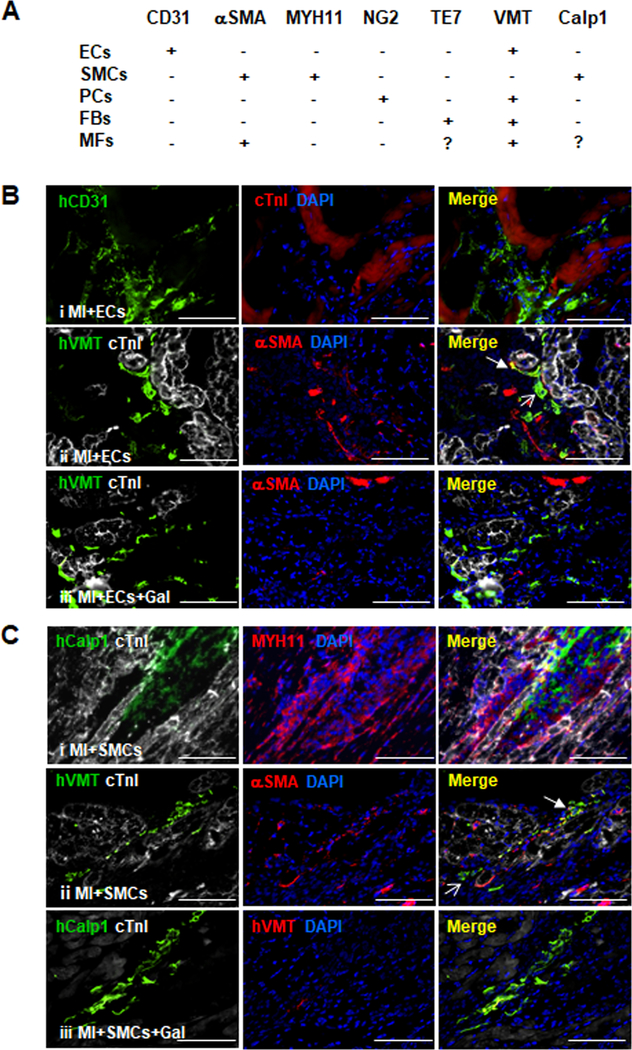
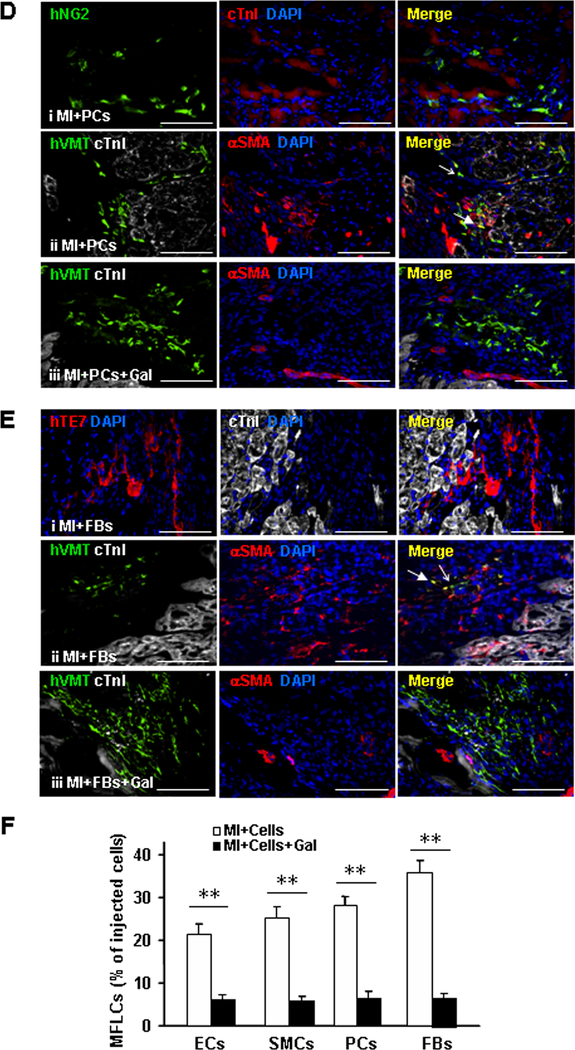
Figure 4. hiPSC-NMCCs can differentiate into myofibroblast-like cells after transplantation into infarcted.
mouse hearts. (A) Marker expression is summarized for ECs, SMCs, pericytes (PCs), fibroblasts (FBs), and myofibroblasts (MFs). (B-F) MI was surgically induced in mice; then, animals were treated with hiPSC-ECs, -SMCs, -pericytes, or -fibroblasts (i.e., the MI+ECs, MI+SMCs, MI+PCs, or MI+FBs groups, respectively) or with hiPSC-ECs, -SMCs, -pericytes, or -fibroblasts and the TGFβ1 inhibitor galunisertib. 7 days later, the mice were sacrificed, and immunofluorescence analyses of marker expression were performed in sections from the site of cell administration. (B) Sections from the MI+ECs group were stained for expression of the human CD31 isoform (hCD31), cTnI, the human isoform of vimentin (hVMT), and αSMA; (C) sections from the MI+SMCs group were stained for expression of the human isoform of calponin 1 (hCalp1), MYH11, hVMT, cTnI, and αSMA; (D) sections from the MI+PCs group were stained for expression of the human isoform of neural/glial antigen 2 (hNG2), hVMT, cTnI, and αSMA; and (E) sections from the MI+FBs group were stained for expression of the human isoform TE7 (hTE7), hVMT, cTnI, and αSMA. Nuclei were counter-stained with DAPI. Examples of cells that continued to express markers of the injected cell lineages or that differentiated to myofibroblasts are identified with open- and closed-headed arrows, respectively. (F) The proportion of hiPSC-derived cells that expressed myofibroblast markers was determined for animals treated with hiPSC-ECs, -SMCs, -pericytes, and -fibroblasts alone (MI+Cells) or with both the hiPSC-derived cells and galunisertib (MI+Cells+Gal) and presented as a percentage (n=6 sections per mouse, 5 mice per group, a minimum of 800 cells from each group were counted). Bar=100 μm; **P<0.01. Two-tailed Student’s t-test for F.