Fig. 7.
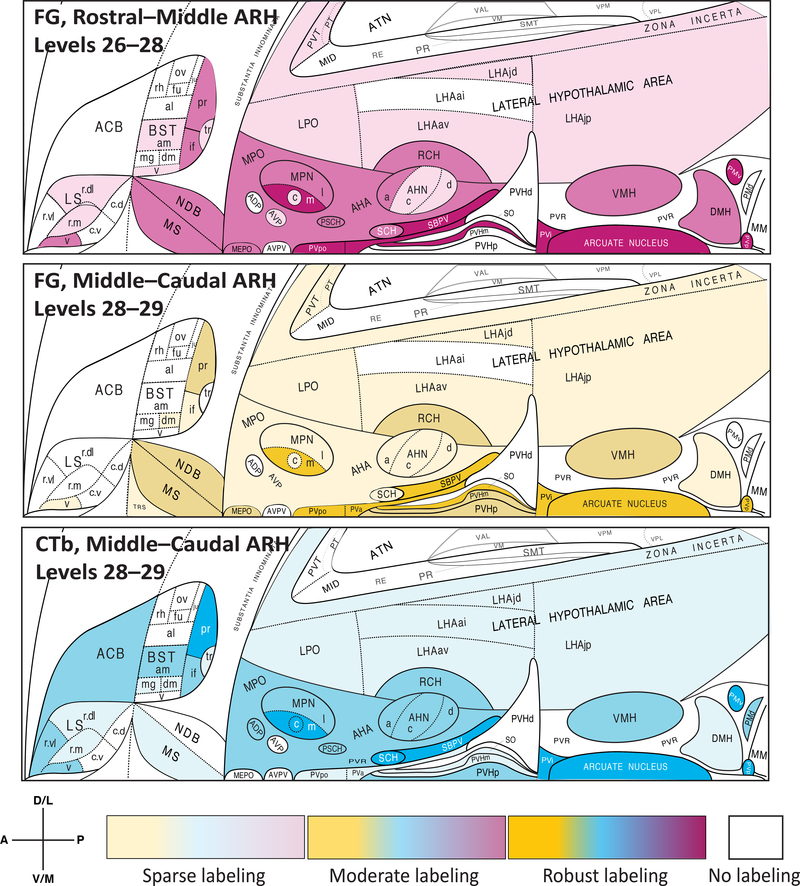
Provisional choropleth flatmaps of unpublished data [268, 269] are furnished here to illustrate differences in the distributions and densities of retrogradely labeled neurons traced from rostral versus caudal locations within the rat ARH using two different retrograde tracers across three test subjects (adult male Sprague-Dawley rats). The upper two panels show distributions and densities for neurons traced retrogradely from ARH injection sites containing deposits of the retrograde tracer, Fluorogold (FG). The bottom (third from top) panel is from a test subject receiving the retrogradely transportable tracer, cholera toxin subunit B (CtB). Note the locations within the ARH of the deposits, which were mapped to specific atlas levels of Swanson (2004) [423] that contain the ARH. The deposit in the case shown in the upper panel was localized to the anterior half (rostral–middle) of the ARH, whereas the lower two panels were from subjects receiving deposits mapped to the posterior third (middle–caudal) of the ARH. Note that at atlas level 28 there was overlap of injection deposits for all three cases. Light to heavy shading for each panel corresponds to sparse to robust numbers of retrogradely labeled cells for each sub-region, with no shading indicating an absence of cells in that region. While many regions show similar densities and distributions of afferent input as would be expected from an overlapping series of injection deposits, there are also clear significant differences in such input to the rostral versus caudal portions of the ARH; most notably, the dorsomedial subdivision of the bed nuclei of the stria terminalis (BSTdm), the central part of the medial preoptic nucleus (MPOc), the periventricular part of the paraventricular hypothalamic nucleus (PVHp), and the dorsal premammillary nucleus (PMd). These differences in afferent distribution and density suggest that ARH recipient neurons are heterogeneous in at least certain functions (and therefore may differ in cellular state or phenotype), underscoring the need for accurate ARH sampling along the rostrocaudal axis of the nucleus for transcriptomic, proteomic or peptidomic studies. The flatmaps from Swanson (2004) [423] (and available at https://larrywswanson.com) are reproduced and modified here under the conditions of a Creative Commons BY-NC 4.0 license (https://creativecommons.org/licenses/by-nc/4.0/legalcode).