Figure 4.
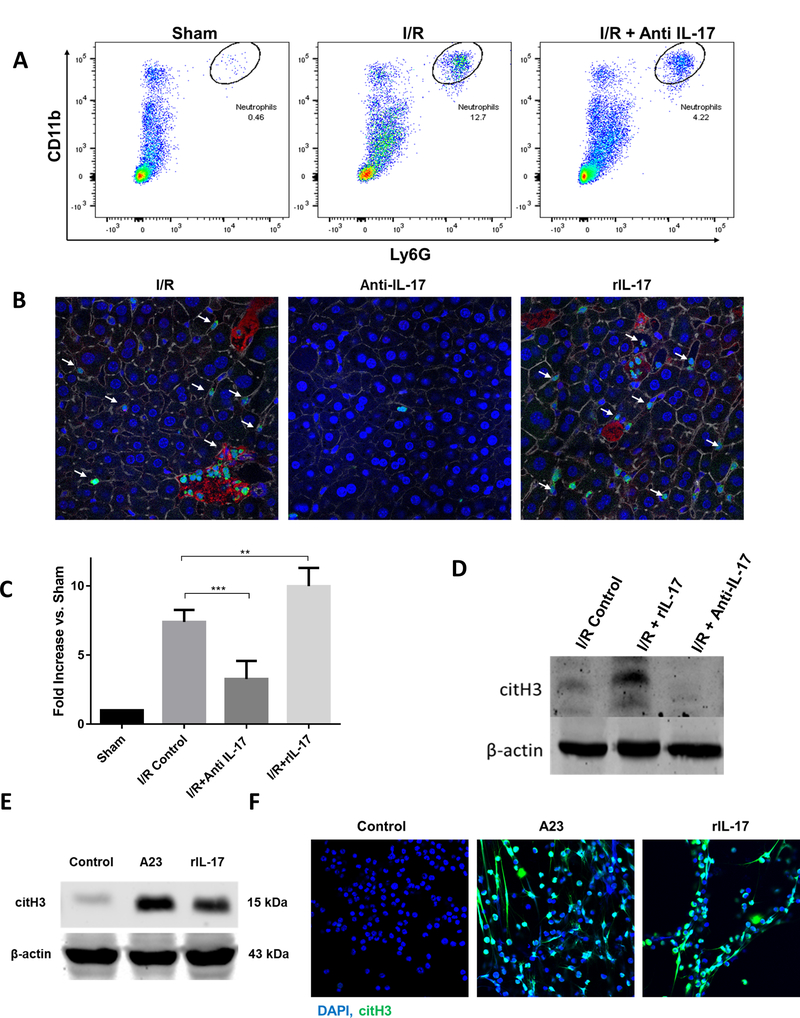
IL-17 increases neutrophil infiltration and neutrophil extracellular trap (NET) formation after I/R. (A) Flow cytometer analysis of liver after ischemia and 6 hour of perfusion reveals increased neutrophil infiltration compared to sham. Neutrophil influx in significantly decreased in mice treated with anti-IL-17. (B) Using confocal microscopy, there is a significant decrease in hepatic infiltrating neutrophils 6 hours hepatic I/R in anti-il-17 treated mice, and an increase in rIL-17 treated mice compared to control mice (mean 2.7 μm2 Ly6G+ area/total cells for control versus 0.7 μm2 Ly6G+ area/total cells for anti-IL-17 mice versus 3.8 μm2 Ly6G+ area/total cells for rIL-17 treated mice, p < 0.001). White arrows indicate neutrophils; Ly6G (green), nuclei (blue), citrullinated histone [citH3] (red), actin (white). Scale Bars 50μm. (C) NETs acutely form in liver tissue 6 hours after liver I/R as assessed by serum levels of MPO-DNA compared to sham. MPO-DNA levels are significantly reduced in mice treated with anti-IL-17 at the time of reperfusion; MPO-DNA levels are increase in livers of mice treated with rIL-17A (D) Cit-H3 protein levels in the liver were determined by Western blot in I/R, I/R + rIL-17, and I/R + anti-IL-17 mice groups (6 per group) 6 hours after liver I/R. Levels of citH3 were elevated when rIL-17 had been given and minimized when anti-IL-17 had been given. (E) In vitro, mouse peripheral derived-neutrophils were treated with A23 (5uM) to induce citrullinated histone [NET formation]. Similarly, citH3 levels were increased when neutrophils were treated with rIL-17 (F) Confocal images show in vitro neutrophils forming NETs when treated with rIL-17 or A23. Nuclei (blue), citH3 (green). Scale Bars 100μm. *P < 0.05, **P<0.01, ***P<0.001.
