Figure 4. Cad6B L-NTF-HA overexpression increases MMP2 activity ex vivo.
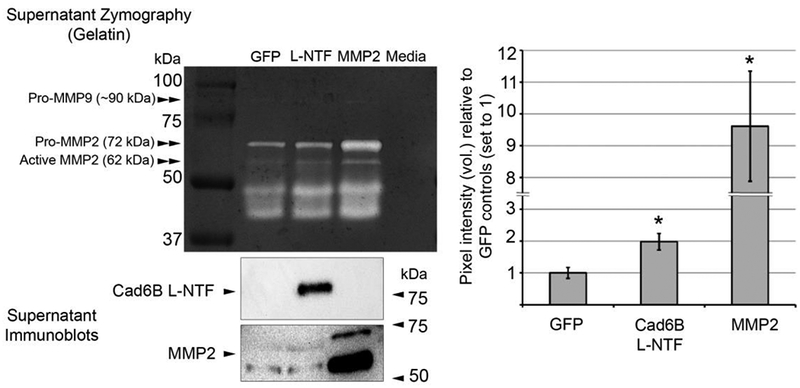
3–4ss embryo neural folds containing premigratory neural crest cells electroporated unilaterally with L-NTF-HA or GFP expression plasmids were excised, pooled, and incubated ex vivo for 48 hours. Conditioned supernatants were collected from the last 24 hours of incubation and tested for recombinant L-NTF and MMP2 expression (immunoblot) and protease activity (gelatin zymography). Immunoblots were performed to ensure that explants were sufficiently electroporated and provide a qualitative, not quantitative, assessment of MMP2 levels after L-NTF or MMP2 overexpression in this assay. Elevated recombinant L-NTF levels in the supernatant resulted in a 2.0 (+/− 0.26)-fold increase in gel degradation at 72 kDa vs. GFP control supernatants, which corresponds to MMP2-mediated degradation. Conditioned supernatants collected from positive control MMP2-overexpressing explants led to a 9.8 (+/− 1.9)-fold increase vs. GFP control supernatants. Asterisks denote a significant difference from GFP (p < 0.05, n = 5). In addition to pro- and active forms of MMP2, faint gelatinase activity was also noted at ~ 90 kDa, indicating possible activity by MMP9. Degraded MMP2 with intact catalytic sites, and possibly other lower molecular weight gelatinases, account for the activity observed between 37 and 50 kDa.
