Figure 6. L-NTFs generated via LMH cell-mediated proteolysis of full-length HA-Cad6B physically associate with recombinant MMP2.
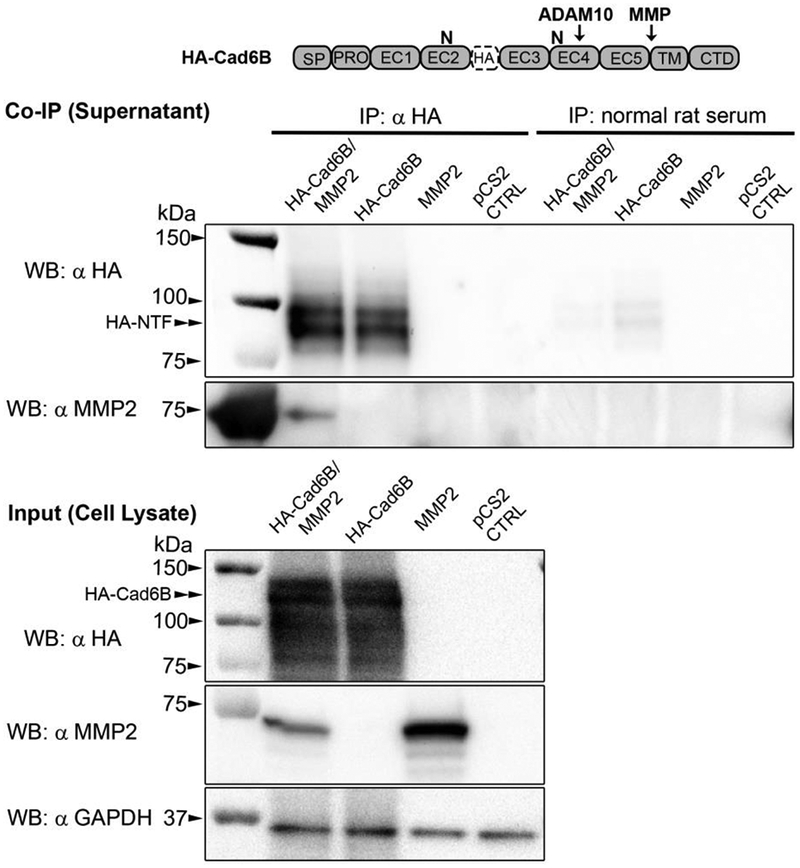
LMH cells were co-transfected with HA-tagged full-length Cad6B and chick MMP2 expression plasmids. Single transfections of HA-Cad6B or MMP2 were also included as co-immunoprecipitation controls. Following overnight transfections, cells were grown in low serum media for an additional 24 hours. Conditioned medias were then subjected to HA antibody pulldown and immunoblotting for HA and MMP2 (serially probed on the same immunoblot). HA immunoblots indicate that cleaved HA-tagged L-NTFs accumulate in the media and are sufficiently immunoprecipitated only in supernatants of cells transfected with HA-Cad6B. No full-length HA-Cad6B was detected in any respective supernatants. Pulldown of HA-tagged L-NTFs from ‘HA-CAD6B only’ control demonstrate that most of the L-NTFs are generated by endogenous LMH proteases (LMH cells express both MMP2 and MMP9, unpublished). Serial immunoblotting with an MMP2 antibody reveals that recombinant 72 kDa MMP2 is co-immunoprecipitated with HA-tagged NTFs. Normal rat serum immunoprecipitations were performed as negative controls for each treatment. Immunoblotting of treatment cell lysates serve as treatment inputs as well as qualitative transfection controls for HA-Cad6B and MMP2. GAPDH immunoblotting was also performed as a loading control between treatments.
