Abstract
Nontargeted GC×GC-TOF/MS analysis of blubber from 8 common bottlenose dolphins (Tursiops truncatus) inhabiting the Southern California Bight was performed to identify novel, bioaccumulative DDT-related compounds and to determine their abundance relative to the commonly studied DDT-related compounds. We identified 45 bioaccumulative DDT-related compounds of which the majority (80%) is not typically monitored in environmental media. Identified compounds include transformation products, technical mixture impurities such as tris(chlorophenyl)methane (TCPM), the presumed TCPM metabolite tris(chlorophenyl)-methanol (TCPMOH), and structurally related compounds with unknown sources, such as hexa- to octachlorinated diphenylethene. To investigate impurities in pesticide mixtures as possible sources of these compounds, we analyzed technical DDT, the primary source of historical contamination in the region, and technical Dicofol, a current use pesticide that contains DDT-related compounds. The technical mixtures contained only 33% of the compounds identified in the blubber, suggesting that transformation products contribute to the majority of the load of DDT-related contaminants in these sentinels of ocean health. Quantitative analysis revealed that TCPM was the second most abundant compound class detected in the blubber, following DDE, and TCPMOH loads were greater than DDT. QSPR estimates verified 4,4′,4″-TCPM and 4,4′4,″-TCPMOH are persistent and bioaccumulative.
Graphical Abstract
INTRODUCTION
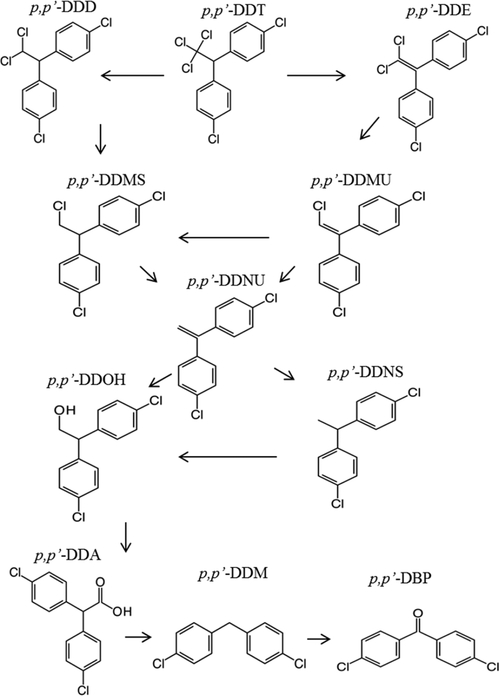
Dichlorodiphenyltrichloroethane (DDT), a technical mixture containing roughly 80% p,p′-DDT and 20% o,p′-DDT, was developed as an insecticide during World War II and, following widespread use and subsequent concern regarding unintended adverse environmental effects, was banned in the U.S. in 1972.1–5 The ultimate fate of DDT in marine systems is closely linked to its transformation, and potential biological impacts are related to the bioaccumulation and biomagnification of both the parent and product compounds. Transformation pathways of DDT have been extensively studied, although not fully elucidated (Figure 1),6–11 and multiple factors contribute to transformation in aquatic environments.12–15 Some breakdown products have known cytotoxic and estrogenic activities (DDMU, DDMS, DDCN, DDA),16 and these and others have been detected in aquatic sediments, surface waters, and fish (DDMU, DDA, DBP, DDPU, DDPS, DDNU, DDNS, DDMS, DDOH, DDM).11,17–21 Due to knowledge gaps in the transformation pathways, the toxicity of the transformation products, and their occurrence in biota, it is unclear if DDT transformation results in a decrease or increase in risk to ecosystems.22
Figure 1.
Proposed transformation pathway of p,p′-DDT, modified from Yu et al. 2011, Eggen et al. 2006, and Jensen et al. 1972.7,9,11
We previously identified halogenated organic compounds (HOCs) in the blubber of an apex predator, common bottlenose dolphins (Tursiops truncatus), inhabiting the Southern California Bight (“Bight”).23 Within the Bight, the Palos Verdes Shelf (PVS) is a Superfund site due to historic contamination of marine sediments by DDT originating from the Montrose Chemical Corporation of California. From the 1950s to the 1970s, the company introduced approximately 1500−2500 t of technical DDT and waste products to the local sewer system, of which roughly half was discharged to the PVS through the outfalls of a wastewater treatment plant.24–26 Nontargeted analysis utilizing comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS) identified 34 classes of HOCs, including chlordane-related compounds, polybrominated diphenyl ethers, halogenated natural products, and several DDT-related contaminants. However, the identity of many of the DDT-related compounds was tentative. GC×GC/TOF-MS provides enhanced chromatographic separation and sensitivity toward collection of full-scan mass spectra, compared to single dimensional GC/MS. Nontargeted analytical frameworks utilizing GC×GC/TOF-MS have proven successful in cataloging HOCs in a variety of environmental samples.23,27–32 Here we present an expanded investigation of the DDT-related compounds.
First, we verified the identities of previously tentatively identified DDT-related compounds in the dolphin blubber samples, reassigned previously unknown mass spectra to DDT-related chemical structures, and quantified the DDT-related compounds. Second, known and potential DDT transformation products were observed in the blubber; however, it was unclear if these were transformation products or were originally present in technical DDT. Third, we analyzed a technical mixture of Dicofol, a current use pesticide synthesized from DDT33,34 which contains DDT-related impurities.35–37 Although DDT content is restricted,38 DDT-related impurities in Dicofol can undergo long-range atmospheric transport,39,40 and it is therefore a potential, although likely minor, source to the region. Fourth, we describe compounds identified in the blubber but not observed in either technical mixture and are likely transformation products. Lastly, we describe compounds found in the blubber that are structurally related to DDE but which contain 6 to 8 chlorines (instead of 4) and are therefore not likely to be degradation products.
MATERIALS AND METHODS
A list of defined acronyms and compound names is provided in the Supporting Information (SI Part A).
Materials.
Residue-analysis grade solvents were used for the extraction and cleanup procedures. The following standards were purchased from AccuStandard (New Haven, CT, USA): 3,3′,4,4′-tetrabromodiphenyl ether (BDE-77); 6-fluoro-2,2′,4,4′-tetrabromodiphenyl ether (6F BDE-47); 4′-fluoro-2,3,3′,4,5,6-hexabromodiphenyl ether (4F BDE-69); p,p′-DDMU; p,p′-DDA; DDT (technical grade); Dicofol aka Kelthane (technical grade); and PBDE Standard Solution for Accuracy and Precision (BDE-28, −47, −99, −100, −153, −154). The following standards were purchased from Wellington Laboratories (Guelph, Canada): 3,3′,4,4′,5,5′-hexachloro-[13C12]biphenyl (13C-PCB-169); 2,3,3′,4,4′,5,5′-heptachloro-[13C12]biphenyl (13C-PCB-189); tris(4-chlorophenyl)methane (TCPM); tris(4-chlorophenyl)methane-13C19 (13C-TCPM), and tris(4-chlorophenyl)methanol (TCPMOH). A custom pesticide standard containing o,p′-DDT, p,p′-DDT, o,p′-DDE, p,p′-DDE, o,p′-DDD, and p,p′-DDD was purchased from Ultra Scientific (N. Kingstown, RI, USA). 4,4′-DBP, 4,4′-bis(4-chlorophenyl) sulfone, and 4,4′-DDM were purchased from Sigma-Aldrich (Milwaukee, WI, USA). 4,4′-DDOH and 4,4-DDNU were obtained from LGC (Manchester, NH, USA).
Collection and Analysis of Dolphin Blubber.
Blubber samples were obtained from 8 sexually mature male bottlenose dolphins (Tursiops truncatus) that inhabit near-shore or deeper off-shore regions of the Bight. All dolphins were fatally stranded on beaches in the region between 1995 and 2010. Stranding and biological information, as well as a detailed description of the procedures for compound identification and relative abundance determination, can be found in our previous study.23
Blubber samples were re-extracted for quantitative analysis using the previous procedures,23 with the exceptions of less sample mass to prevent chromatographic overloading and utilization of a larger set of internal standards. Method details are provided in the SI Part A. Briefly, dolphin blubber samples were extracted using pressurized liquid extraction (ASE 300, Dionex, Sunnyvale, CA, USA) with dichloromethane. To reduce contaminant exclusion or transformation during cleanup, a one-step gel permeation chromatography (GPC, J2 Scientific, Columbia, MO) procedure was used to remove lipids. Samples were analyzed using a Pegasus 4D (LECO, St. Joseph, MI, USA) time-of-flight mass spectrometer with electron ionization mode, connected to an Agilent 6890 gas chromatograph (Palo Alto, CA, USA) (Table S1). Data analysis was performed with LECO ChromaTOF software version 4.43.3 utilizing automatic peak finding and mass spectral deconvolution.
Confirmation of DDT-Related Compounds.
Note both the use of a blubber matrix and the GC-based instrumentation result in preferentially detecting nonpolar compounds as opposed to polar compounds. The identities of some compounds were verified with authentic standards, some previously classified as only “DDT-related” were assigned specific structures,23 and some previously unknown spectra were also assigned structures. For example, compounds previously identified as DDT-related 1, 2, 8, and 9 were further characterized in this work as follows. An authentic standard of p,p′-DDM matched the retention time and mass spectrum of DDT-related 2. The mass spectra for compounds previously assigned as DDT-related 1, 8, and 9 also matched the p,p′-DDM reference standard, but the retention times did not match. Therefore, these compounds are likely isomers of p,p′-DDM and were tentatively assigned as DDM 1, 2, and 3 (Table 1). Identifications were made through one of the following categories and are listed in Table 1: [1] The experimental mass spectrum and retention time were matched to those of a reference standard analyzed under the same conditions. [2] The experimental spectrum, but not the retention time, was matched to a reference standard, indicating the presence of an isomer. [3] The experimental spectrum was matched to one within the technical DDT mass spectral library provided in the SI. [4] The experimental mass spectrum was identified as potentially belonging to a class of congeners on the basis of comparison to a reference standard within the same class of congeners. Additionally, identifications in categories [2] and [4] must be aligned in 2D chromatographic space with the corresponding authentic standards. The mass spectra, fragment ion identifications, and retention times are reported SI Part B along with the corresponding authentic standard mass spectra.
Table 1.
DDT-Related Compounds Identified in Southern California Bottlenose Dolphin Blubber Samples (n = 8)a
| compound | DDT tech. | Dicofol tech. | transformation | identificationb |
|---|---|---|---|---|
| DDTs and Major Transformation Products That Are Typically Monitored | ||||
| DDT | ||||
| p,p′ -DDT | x | x | parent | [1] |
| o,p′ -DDT | x | x | parent | [1] |
| DDE | ||||
| p,p′ -DDE | x | x | x | [1] |
| p,p′ -DDE | x | x | x | [1] |
| DDD | ||||
| p,p′-DDD | x | x | [1] | |
| o,p′ -DDD | x | [1] | ||
| DDMU | ||||
| p,p′-DDMU | x | x | [1] | |
| DDMU 1 | x | [2] | ||
| DDMU 2 | x | x | x | [2] |
| DDT Transformation Products That Are Not Typically Monitored | ||||
| DDM | ||||
| p,p′-DDM | x | x | x | [1] |
| DDM 1 | x | x | [2] | |
| DDM 2 | x | [2] | ||
| DDM 3 | x | [2] | ||
| DBP | ||||
| p,p′ -DBP | x | x | x | [1] |
| DDNS | ||||
| DDNS | x | x | [3] | |
| DDNU | ||||
| p,p′-DDNU | x | x | x | [1] |
| DDOH | ||||
| DDOH 1 | x | [2] | ||
| DDOH 2 | x | [2] | ||
| Hexa- to Octachlorinated Diphenylethene | ||||
| DDE 6Cl 1 | [4] | |||
| DDE 6Cl 2 | [4] | |||
| DDE 6Cl 3 | [4] | |||
| DDE 6Cl 4 | [4] | |||
| DDE 7Cl 1 | [4] | |||
| Hexa- to Octachlorinated Diphenylethene | ||||
| DDE 7Cl 2 | [4] | |||
| DDE 7Cl 3 | [4] | |||
| DDE 8Cl | [4] | |||
| TCPM and TCPMOH Related Compounds | ||||
| TCPM | ||||
| 4,4′,4”-TCPM | [1] | |||
| TCPM 1 | [2] | |||
| TCPM 2 | [2] | |||
| TCPM 3 | x | [2] | ||
| TCPM 4 | [2] | |||
| TCPM 4Cl 1 | [4] | |||
| TCPM 4Cl 2 | [4] | |||
| TCPM 4Cl 3 | [4] | |||
| TCPM 5 | x | [2] | ||
| TCPM 6 | [2] | |||
| TCPM 7 | [2] | |||
| TCPMOH | ||||
| 4,4′,4”-TCPMOH | [1] | |||
| TCPMOH 1 | [2] | |||
| TCPMOH 2 | [2] | |||
| TCPMOH 3 | [2] | |||
| TCPMOH 4 | [2] | |||
| TCPMOH 5 | [2] | |||
| TCPMOH 4Cl | [4] | |||
| Other DDT-Related Compounds | ||||
| DDT related 1 | x | [3] | ||
Compounds that were also detected in technical DDT, Dicofol, or reported as a microbial transformation products of DDT are denoted by an “x”.
Identification uncertainty categories are defined in the Materials and Methods section. Mass spectra and ancillary information associated with identification (i.e. GC×GC retention times, mass fragments, and corresponding standard mass spectrum) are available in SI Part B.
Quantitation of DDT-Related Compounds.
Quantified compounds (Table S2) include both typically monitored and nonmonitored transformation products and related compounds such as the TCPM and TCPMOH isomers. Newly extracted blubber samples with masses of either 0.1 g of lipid or 1 g of lipid were quantified by GC×GC/TOF-MS. For compounds that were chromatographically overloaded, such as p,p′-DDE, the smaller sample size (0.1 g of lipid) was used for quantitation. Samples were injected in the hot splitless mode (2 μL, 300 °C) into a Restek (Bellefonte, PA) Rtx5-MS column (30-m length, 0.25 mm i.d., 0.25-μm film thickness) with a 5 m guard column, followed by a Restek Rxi-17 column (1-m length, 0.10 mm i.d., 0.10-μm film thickness). Instrumental conditions were the same as our previous analysis.23 The full GC temperature program is described in the SI (Table S1). Quantitative analysis was performed using multipoint calibration curves. If an exact authentic standard could not be obtained, quantitation was performed using a standard of an isomer or structurally related compound. Manual reviews of all compound identifications and peak integrations were performed to ensure accuracy. The average recovery of the BDE 77, 13C-TCPM, and 6F BDE-47 internal standards used in the quantitative analysis was 65% ± 21 and 64% ± 21 for the 1 g lipid and 0.1 g lipid samples, respectively. Procedural blanks were prepared and analyzed in the same manner as the blubber samples. All blubber samples had levels at least three times greater than those of the procedural blanks. Polybrominated diphenyl ethers (PBDEs, BDE-28, BDE-47, BDE-99, BDE-100, BDE-153, and BDE-154) were also quantified for comparison against the DDT-related compound concentrations (Table S3).
Analysis of Technical Pesticide Mixtures.
Solutions of 1, 10, 100, and 500 μg/mL technical grade DDT and Dicofol were analyzed by GC×GC/TOF-MS, using the same instrumental conditions described above and in Table S1. As expected, a larger number of components were detected using the 500 μg/mL solutions (Figure S1); therefore, this concentration was used to characterize the technical mixtures and determine the presence or absence of corresponding compounds in the blubber samples. For both technical mixtures, the mass spectra and ancillary information (e.g., retention times and fragment ion identifications) for all identified components were stored in custom mass spectral libraries. The libraries are available as PDF reports in the SI or at http://OrgMassSpec.github.io as text files in the NIST MSP format for import into other software (library names SpecLibDDT2015 and SpecLibKelthane2015). The uncertainty in each identification was categorized based on the method described above.
Quantitative Structure Property Relationships (QSPRs).
For comparative purposes, selected physical and chemical properties of TCPM and TCPMOH were estimated using the QSPRs provided by the EPI (Estimation Programs Interface) Suite software v 4.10.41 The properties evaluated include octanol−water partition coefficients (log Kow), water solubility, biodegradability, and bioconcentration factors (BCF). These are the properties outlined by Muir and Howard to determine if a compound is persistent and bioaccumulative.42–44
RESULTS AND DISCUSSION
Identification of DDT-Related Compounds in Dolphin Blubber.
Forty-five DDT-related compounds were identified in the dolphin blubber samples including the 6 major DDT isomers, breakdown products, related compounds with higher chlorine content compared to DDT, and TCPM-related compounds (structures are shown in Figure 2). Table 1 lists all compounds, the category of identification, and whether they are transformation products. Mass spectra and ancillary information associated with identification are available in SI Part B. The majority (80%) of the compounds is not typically monitored in environmental media. For comparison, Table 1 also lists compounds found in both the blubber and the two technical mixtures, as discussed in the next section.
Figure 2.
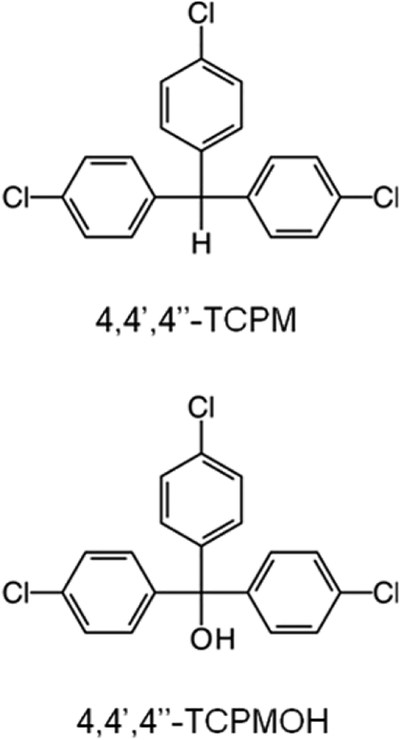
Chemical structures for tris(4-chlorophenyl)methane (TCPM) and tris(4-chlorophenyl)methanol (TCPMOH).
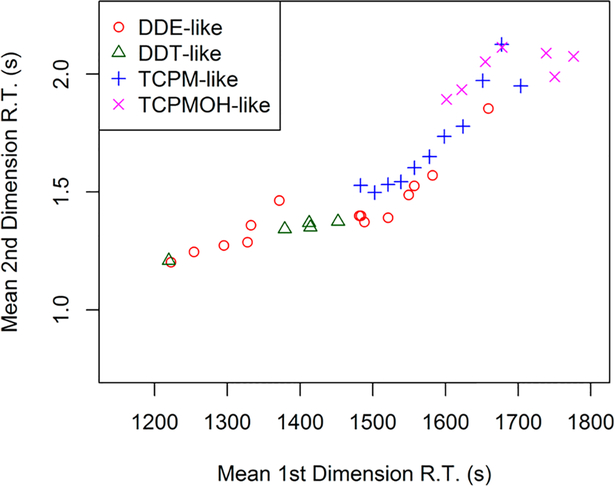
Twenty-six percent of the compounds were identified using authentic standards (providing the highest confidence in the identification). For the tentatively identified compounds, retention times provided additional confidence in the identification, since related congeners and isomers tend to align in this column combination (with Rtx-5 ms as the primary column and Rxi-17 as the secondary column).27 Compounds sharing the same structural backbone were plotted to examine the retention time alignment. For example, the DDE-like compounds, including isomers of DDE, DDNU, DDMU, and highly chlorinated DDEs (hexa- to octachlorinated diphenylethene) are shown in Figure 3. These compounds aligned in 2-D chromatographic space, and increasing chlorination resulted in increasing retention times. For example, the hexa- to octachlorinated diphenylethene eluted later than DDE.
Figure 3.
Two-dimensional (GC×GC) retention times of the DDT-related compounds grouped by structural class. Retention times are an average of the 8 samples. DDE-like refers to DDE, DDNU, and DDMU isomers and hexa- to octachlorinated diphenylethene. DDT-like refers to DDT, DDD, DDNS, and DDMS isomers. TCPM-like refers to isomers with the same backbone structure containing either 3 or 4 chlorines. TCPMOH-like refers to isomers with the same backbone structure containing either 3 or 4 chlorines.
Well Known DDTs.
The p,p′- and o,p′- isomers of DDT and its primary transformation products DDE and DDD were identified in the dolphin blubber, all of which were confirmed with authentic standards. These compounds have been the primary focus of monitoring45–47 because they are persistent and bioaccumulative in wildlife and have been shown to exhibit toxicity.48–54 The major transformation products are formed under both aerobic conditions (p,p′-DDE) and anaerobic conditions (p,p′-DDD).11 The reductive dechlorination product of DDE, DDMU, was also detected in the blubber (p,p′-DDMU was confirmed by authentic standard; 2 other isomers were also detected).55 In 2008, p,p′-DDMU was added to Bight monitoring surveys after investigators determined its presence in PVS sediments, and it was found that on average p,p′-DDMU was 5% of the total concentration of DDT.24 One notable exception to this average was a sample near the PVS margin where, in addition to containing the highest concentrations of DDT, DDMU was nearly 50% of the total DDT concentration.46 Following this survey, p,p′-DDMU was detected and monitored in the water column throughout the PVS.56 Recently, p,p′-DDNU has also been detected in PVS sediments and water, although it is not routinely monitored.57,58 Further research is required to determine whether the transformation products detected in the dolphin blubber samples originate from PVS sediments.
Nonmonitored Transformation Products.
Previous transformation pathway investigations provided insight on the origins of additional DDT-related compounds (Figure 1).6–11,55 Among these, we identified the following in dolphin blubber: p,p′-DDM and 3 other isomers; p,p′-DBP; DDNS; p,p′-DDNU; and DDOH (2 isomers). All compounds for which a specific isomer is assigned (e.g., p,p′-) were confirmed with authentic standards. Although not typically monitored in contaminant surveys, these HOCs were previously detected in sediments from China11 and surface waters from Germany.18,20 Both study regions had heavy impacts from industrial activities, and the German study area was home to a DDT production facility. While there is limited data on the occurrence of these transformation products in marine mammals, the findings of the current study indicate these compounds bioaccumulate and potentially biomagnify in marine food webs.
TCPM or TCPMOH Related Compounds.
A total of 18 compounds belonging to this class were identified in the dolphin blubber samples: 4,4′,4″-TCPM and 7 other isomers, TCPM 4Cl (3 isomers), 4,4′,4″-TCPMOH and 5 other isomers, and TCPMOH 4Cl (Figure 2 and Table 1). An authentic standard was used for confirmation of all compounds for which a specific isomer was assigned (SI Part B). The primary source of TCPM and TCPMOH in the environment is largely unknown, despite both contaminants being present on a global scale.59,60 TCPMOH was initially detected in the late 1980s in harbor seals from the northwestern United States,61 followed by its presumed precursor TCPM in ringed seals from the Baltic Sea in 1992.62 Possible origins of TCPM in these reports include production of synthetic polymers and lightfast dyes and its presence as a trace byproduct of technical organochlorines such as DDT and Dicofol.59,60 Significant correlations between both TCPM and TCPMOH with DDTs in animals feeding at high trophic levels have been reported.63 Further evidence linking TCPM to DDT was published in 2010 by Holmstrand et al., in which the δ37Cl value for TCPM closely resembled that of technical DDT, suggesting TCPM is of anthropogenic origin.64 Our analysis detected a number of TCPM and TCPMOH isomers with a varying degree of chlorination, which have not been previously reported.
Hexa- to Octachlorinated Diphenylethene.
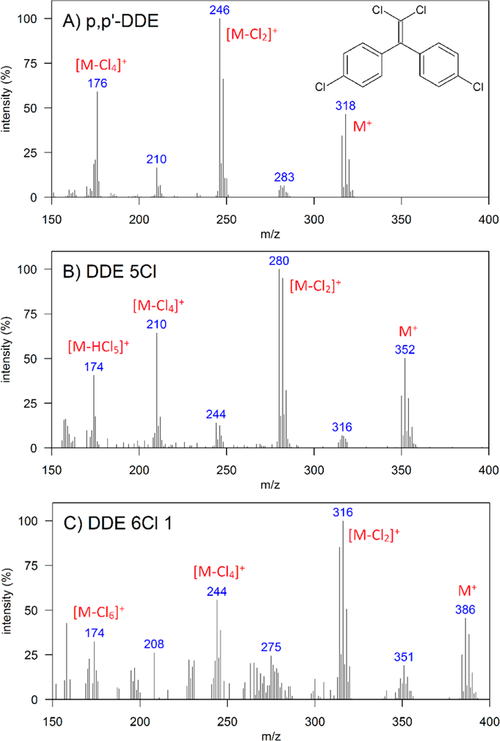
Eight compounds detected in the dolphin blubber are suspected to contain the diphenylethene DDE backbone but with six to eight chlorines instead of the four chlorines on DDE (Table 1). Due to the lack of authentic standards, all eight were identified based on similarity to the fragmentation pattern of p,p′-DDE, in particular the presence of [M+], [M − 2Cl]+, and [M − 4Cl]+ (SI Part B). As supporting evidence for these identifications, we also identified a pentachlorinated diphenylethene within the p,p′-DDE standard solution (Figure 4). This impurity had a fragmentation pattern similar to that of p,p′-DDE but with the molecular ion m/z corresponding to the addition of a fifth chlorine. This compound is likely to be a pentachlorinated diphenylethene (Figure 4), and its presence in a standard solution indicates it may be a synthesis byproduct. Literature data on these compounds is sparse, and they were not observed in the technical mixtures discussed below.
Figure 4.
Mass spectra obtained by GC×GC/TOF-MS of a) p,p′-DDE standard, b) impurity within the p,p′-DDE standard solution tentatively identified as pentachlorinated diphenylethene, and c) an isomer of hexachlorinated diphenylethene, DDE 6Cl 1, found in southern California bottlenose dolphin blubber. “M” indicates the molecular ion.
Other DDT-Related Compounds.
DDT-related 1 (Table 1 and SI Part B) was identified in both the dolphin blubber and the DDT technical mixture. While it has a spectrum identical to p,p′-DDMU, the retention times did not match and the structure is therefore unknown.
Technical DDT and Dicofol as Potential Sources for DDT-Related Compounds.
The production of technical DDT results in leftover starting material, byproducts, and/or impurities. These additional compounds may also be environmental contaminants of interest.43 Although limited compared to our instrumentation, previous studies characterized the components of technical DDT, many of which are known transformation products of DDT, such as DDE, DDD, and DDOH.59,65,66
DDT Technical Mixture.
A total of 89 chlorinated organic compounds were detected in the DDT technical mixture. Forty were identified as either an exact or isomeric match to authentic standards: DDT (o,p′-, p,p′,- and 2 other isomers), DDE (o,p′-, p,p′- ,and 3 other isomers), DDD (o,p′-, p,p′-, and 2 other isomers), DDMU (p,p′- and 15 compounds sharing the identical mass spectrum), DDM (p,p′- and 1 other isomer), DDNS, DDNU (p,p′-), DBP (o,p′-, p,p′-), DDOH (1 isomer), TCPM (2 isomers), and dichlorodiphenyl sulfone (p,p′- and one other isomer). Twenty-five compounds were identified through a NIST library match only (compound classes: chlorobenzaldehyde, trichloroethenylbenzene, trichloro-(chlorophenyl)ethane, trichloro(propenyl)benzene, and pentachloroethylbenzene). The remaining 24 compounds were identified as unknowns with 10 classes based on mass spectral similarity. The mass spectra for all compounds are in the SI library; only those that are also in the dolphin blubber are listed in Table 1. The relatively large number of 16 DDMU related compounds in the technical mixture was in contrast to the 3 isomers detected in the dolphin blubber. DDT and its major transformation products DDD and DDE were all previously identified in technical DDT.59,65,66 The compound p,p′-dichlorodiphenyl sulfone was previously detected in a DDT technical product analyzed by Haller et al.66
Detection of TCPM in the technical mixture provides evidence of a link between this compound and technical DDT. This was initially reported by Buser et al. in 1995, who discovered three TCPM isomers (4,4′4″-, 2,2′4″-, and 2,4′,4″-TCPM) in commercial DDT.59 We did not detect 4,4′,4″-TCPM in the technical DDT; however, 2 other isomers present in the dolphin blubber were detected in the technical product. Our analysis was limited to one product, and it is possible others contain 4,4′,4″-TCPM.
Technical Dicofol.
Dicofol (trade name Kelthane, 2,2,2,-trichloro-1,1-bis(4-chlorophenyl)ethanol, Figure S2) is a current use acaricide synthesized from DDT.33,34,36 Qiu et al., among others, reported the presence of DDT-related impurities such as o,p′-DDT, o,p′-DDE, p,p′-DDT, and p,p′- DDT with an additional chlorine in technical Dicofol.35–37 These impurities may be an additional source of DDTs to the region through long-range atmospheric transport followed by deposition.38–40,67–69
A total of 22 chlorinated organic compounds were identified in the technical Dicofol. The following were identified based on exact or isomeric matches to authentic standards: DDT (o,p′-, p,p′-), DDE (o,p′-, p,p′-, and 3 other isomers), DDMU (1 isomer), DDM (p,p′-), DBP (o,p′-, p,p′-, and 2 other isomers), and DDOH (1 isomer) (Table 1). Additionally, there was one compound identified through the NIST library, chlorodiphenylmethanone, and 7 unknown compounds with 2 classes based on mass spectral similarity. The active ingredient 2,2,2-trichloro-1,1-bis(4-chlorophenyl)ethanol (Figure S2) was not detected, likely due to its rapid transformation to p,p′-DBP within high temperature GC-inlet systems.70 All compounds identified in technical Dicofol were also identified in technical DDT; therefore, dolphin exposure to the two mixtures could not be distinguished.
Technical Mixture Comparison to Dolphin Blubber.
Fifteen compounds were identified in both the blubber and technical DDT, whereas 8 were identified in both the blubber and technical Dicofol (Table 1). These compounds included typically monitored DDT and breakdown products DDE, DDD, and DDMU; nonmonitored breakdown products DDM, DBP, DDNS, and DDNU; and TCPM. A number of compounds (p,p′-DDMU, p,p′-DDD, TCPM 5, o,p′-DDD, p,p′-DBP, TCPM 3, and DDNS) with relatively high concentrations in the blubber were also detected in the technical mixtures. Out of the 45 DDT-related compounds identified in the blubber, 16 correspond to DDT breakdown products, with 10 of the 16 also identified in the technical mixtures. Note there is also a potential difference between the historical discharge to the region and the technical mixtures used in this study.
Quantification of DDT-Related Compounds in Dolphin Blubber.
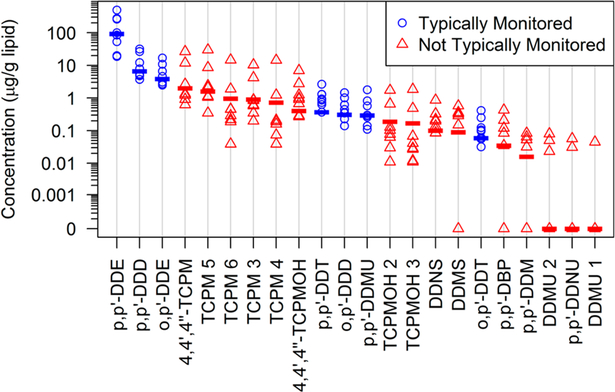
Lipid-normalized concentrations of the 22 most abundant and frequently detected compounds were quantified (Figure 5 and Table S2). The average total concentration of DDT and related compounds in the dolphin samples was 207 ± 62 μg/g lipid. As expected, p,p′-DDE was the most dominant contaminant, followed by p,p′-DDD, o,p′-DDE, five TCPM isomers, and 4,4′,4″-TCPMOH. The total average concentration of the five TCPM isomers was 19 ± 10 μg/g lipid, and this was the second most abundants class, following total DDE (171 ± 60 μg/g lipid). Total TCPMOH concentrations (2 ± 1 μg/g lipid) were greater than total DDT (1.2 ± 0.3 μg/g lipid). The relatively large number TCPM and TCPMOH isomers contributed to their elevated total concentrations. The two most abundant compounds were 4,4′,4″-TCPM and 4,4′4,″-TCPMOH, at 6.0 ± 3.0 and 1.8 ± 0.8 μg/g lipid, respectively. These concentrations were higher than those measured in adult male sea lions from the California coast (including the Bight) of 2.29 ± 3.25 and 0.44 ± 0.37 μg/g lipid, respectively.71 TCPM, TCPMOH, DDMS, and DDNS levels detected in the dolphin blubber were comparable to those of the routinely monitored PBDEs (Table S2 and Table S3).
Figure 5.
Lipid-normalized concentrations of the 22 most abundant and frequently detected compounds in southern California bottlenose dolphins. Symbols represent individual samples (n = 8), and the dash represents the median. Blue dots indicate “typically monitored DDT compounds”, and red triangles indicate “not typically monitored DDT compounds” respectively. The acronyms are listed in the SI Part A.
According to Howard and Muir, a compound is predicted to be persistent and bioaccumulative if the following criteria are met: log Kow greater than 5, bioconcentration factor (BCF) greater than 2000, and low biodegradability.42 The Biode-gradation Probability Program (BIOWIN) within the EPI Suite program estimated the probability an organic chemical will rapidly biodegrade in the presence of mixed populations of environmental microorganisms using six different models.72–76 The following QSPR estimates were obtained for 4,4′,4″-TCPM and 4,4′4,″-TCPMOH, respectively: Log Kow of 7.30 and 6.31, BCF of 9483 and 6802, and BIOWIN estimates showing persistence (low biodegradability) for both compounds.41 These estimated properties provide supporting evidence that 4,4′,4″-TCPM and 4,4′4,″-TCPMOH are persistent and bioaccumulative in environmental media.
The bioaccumulation of HOCs, including DDT-related compounds, by marine mammals is known to be species, gender, and age/sexual maturity specific.77 Therefore, comparisons to other surveys were made only for Tursiops truncatus and Tursiops aduncus adult males (Table S4). In some cited cases, the age/sexual maturity of male dolphins was not reported. In addition, comparisons were only made for data collected during the years 1995 to 2015. On average, ∑6DDT was 184 μg/g lipid and ∑4DDTs was 178 μg/g lipid (n = 8) for the blubber samples quantified in this study, where ∑6DDT is the sum of the p,p′- and o,p′- DDT, DDD, and DDE isomers, and ∑4DDT is the sum of p,p′-DDT, p,p′-DDD, p,p′-DDE, and o,p′-DDT. These concentrations are an order of magnitude higher than bottlenose dolphin values reported elsewhere within the United States and globally (Table S4). This suggests that marine mammals frequenting the Bight may have among the highest DDT-related HOC concentrations worldwide.
Implications.
This work established the bioaccumulation of DDT transformation products and technical mixture impurities in bottlenose dolphins beyond those that are known to occur and normally monitored. It is a step in further understanding of the fate of these compounds, and more broadly DDT, in marine environments. The concentrations of typically monitored DDT compounds accumulating in the bottlenose dolphins from the southern California Bight were at least 1 order of magnitude higher than values reported from other regions. TCPM, a largely unstudied compound, was the second most abundant class in the dolphins, following DDE. This work informs bioaccumulation and toxicological assessments of marine life where DDE is not the only DDT-related compound of concern.
Recommendations for future work specific to the chemical analysis of the nonmonitored DDT-related compounds observed in this study, with the highest priority given to TCPM-related compounds, include (1) measurement of their occurrence and spatial distribution; (2) quantification in sediment and biota at different trophic levels to investigate persistence and biomagnification; and (3) analysis of dated sediment cores to understand historical input and transformation rates in coastal marine environments such as the PVS.
Supplementary Material
ACKNOWLEDGMENTS
We thank Miles Borgen (University of California San Diego), Kayo Watanabe (San Diego State University Research Foundation), and Wayne Lao (Southern California Coastal Water Research Projects) for their chemical and data analysis assistances; Jim Dines (Natural History Museum of Los Angeles County) for providing samples; and Nicky Beaulieu, John Heyning, Dave Janiger, and Kelly Robertson for collection of samples in the field.
Funding
This research was funded by the National Science Foundation (OCE-1313747) and National Institute of Environmental Health Sciences (P01-ES021921) through the Oceans and Human Health Program, the California State University Program for Education and Research in Biotechnology (CSUPERB), and the member agencies of SCCWRP.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b03150.
Glossary of acronyms, two supplemental figures, and four tables (Part A) (PDF)
Information associated with identification of the 45 DDT-related compounds (mass spectra, interpretation of mass spectral fragmentation patterns, chromatographic retention times, and corresponding standards’ mass spectra) (Part B) (PDF)
Mass spectral library report for DDT technical mixture (PDF)
Mass spectral library report for Dicofol technical mixture (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Anderson DW; Jehl JR Jr.; Risebrough RW; Woods LA Jr.; Deweese LR; Edgecomb WG Brown pelicans. Improved reproduction off the southern California coast. Science 1975, 190 (4216), 806–8. [DOI] [PubMed] [Google Scholar]
- (2).Fry DM; Toone CK DDT-induced feminization of gull embryos. Science (Washington, DC, U. S.) 1981, 213 (4510), 922–4. [DOI] [PubMed] [Google Scholar]
- (3).Kale SP; Murthy NBK; Raghu K; Sherkhane PD; Carvalho FP Studies on degradation of 14C-DDT in the marine environment. Chemosphere 1999, 39 (6), 959–968. [DOI] [PubMed] [Google Scholar]
- (4).Megharaj M; Kantachote D; Singleton I; Naidu R Effects of long-term contamination of DDT on soil microflora with special reference to soil algae and algal transformation of DDT. Environ. Pollut. (Oxford, U. K.) 2000, 109 (1), 35–42. [DOI] [PubMed] [Google Scholar]
- (5).Sharpe RM Reproductive biology: Another DDT connection. Nature (London, U. K.) 1995, 375 (6532), 538–9. [DOI] [PubMed] [Google Scholar]
- (6).Aislabie JM; Richards NK; Boul HL Microbial degradation of DDT and its residues - a review. N. Z. J. Agric. Res 1997, 40 (2), 269–282. [Google Scholar]
- (7).Eggen T; Majcherczyk A Effects of zero-valent iron (Fe0) and temperature on the transformation of DDT and its metabolites in lake sediment. Chemosphere 2006, 62 (7), 1116–1125. [DOI] [PubMed] [Google Scholar]
- (8).Huang HJ; Liu SM; Kuo CE Anaerobic biodegradation of DDT residues (DDT, DDD, and DDE) in estuarine sediment. J. Environ. Sci. Health, Part B 2001, 36 (3), 273–88. [DOI] [PubMed] [Google Scholar]
- (9).Jensen S; Gothe R; Kindstedt M Bis-(p-chlorophenyl)-acetonitrile (DDCN), a new DDT derivative formed in anaerobic digested sewage sludge and lake sediment. Nature 1972, 240, 421–422. [Google Scholar]
- (10).Sayles GD; You G; Wang M; Kupferle MJ DDT, DDD, and DDE Dechlorination by Zero-Valent Iron. Environ. Sci. Technol 1997, 31 (12), 3448–3454. [Google Scholar]
- (11).Yu H-Y; Bao L-J; Liang Y; Zeng EY Field Validation of Anaerobic Degradation Pathways for Dichlorodiphenyltrichloroethane (DDT) and 13 Metabolites in Marine Sediment Cores from China. Environ. Sci. Technol 2011, 45 (12), 5245–5252. [DOI] [PubMed] [Google Scholar]
- (12).Garrison AW; Nzengung VA; Avants JK; Ellington JJ; Jones WJ; Rennels D; Wolfe NL Phytodegradation of p,p′-DDT and the Enantiomers of o,p′-DDT. Environ. Sci. Technol 2000, 34 (9), 1663–1670. [Google Scholar]
- (13).Gaw SK; Palmer G; Kim ND; Wilkins AL Preliminary evidence that copper inhibits the degradation of DDT to DDE in pip and stonefruit orchard soils in the Auckland region, New Zealand. Environ. Pollut. (Oxford, U. K.) 2003, 122 (1), 1–5. [DOI] [PubMed] [Google Scholar]
- (14).Llompart M; Lores M; Lourido M; Sanchez-Prado L; Cela R On-fiber photodegradation after solid-phase microextraction of p,p′-DDT and two of its major photoproducts, p,p′-DDE and p,p′-DDD. J. Chromatogr., A 2003, 985 (1–2), 175–183. [DOI] [PubMed] [Google Scholar]
- (15).Van Zwieten L; Ayres MR; Morris SG Influence of arsenic co-contamination on DDT breakdown and microbial activity. Environ. Pollut. (Oxford, U. K.) 2003, 124 (2), 331–339. [DOI] [PubMed] [Google Scholar]
- (16).Wetterauer B; Ricking M; Otte JC; Hallare AV; Rastall A; Erdinger L; Schwarzbauer J; Braunbeck T; Hollert H Toxicity, dioxin-like activities, and endocrine effects of DDT metabolites-DDA, DDMU, DDMS, and DDCN. Environ. Sci. Pollut. Res 2012, 19 (2), 403–415. [DOI] [PubMed] [Google Scholar]
- (17).Heberer T; Duennbier U DDT Metabolite Bis-(Chlorophenyl)acetic Acid: The Neglected Environmental Contaminant. Environ. Sci. Technol 1999, 33 (14), 2346–2351. [Google Scholar]
- (18).Kronimus A; Schwarzbauer J; Ricking M Analysis of Non-Extractable DDT-Related Compounds in Riverine Sediments of the Teltow Canal, Berlin, by Pyrolysis and Thermochemolysis. Environ. Sci. Technol 2006, 40 (19), 5882–5890. [DOI] [PubMed] [Google Scholar]
- (19).Schwarzbauer J; Ricking M; Franke S; Francke W Organic compounds as contaminants of the Elbe River and its tributaries. Part 5. Halogenated organic contaminants in sediments of the Havel and Spree Rivers (Germany). Environ. Sci. Technol 2001, 35 (20), 4015–4025. [DOI] [PubMed] [Google Scholar]
- (20).Schwarzbauer J; Ricking M; Littke R DDT-Related Compounds Bound to the Nonextractable Particulate Matter in Sediments of the Teltow Canal, Germany. Environ. Sci. Technol 2003, 37 (3), 488–495. [DOI] [PubMed] [Google Scholar]
- (21).Yang W; Yu Z-Q; Xiang-Fan L; Jia-Liang F; Dong-Ping Z; Guo-Fa R; Guo-Ying S; Jia-Mo F Qualitative analysis of some emerging halogenous pollutions in fish sample by comprehensive twodimentional gas chromatography/time-of-flight mass spectrometry. Chinese Journal of Anlytical Chemistry 2012, 40 (8), 1187–1193. [Google Scholar]
- (22).EPA US Final Palos Verdes Shelf Superfund Site Remedial Investigation Report; U.S. Environmental Protection Agency: Region 9, San Francisco, CA: 94105, October 2007. [Google Scholar]
- (23).Shaul NJ; Dodder NG; Aluwihare LI; Mackintosh SA; Maruya KA; Chivers SJ; Danil K; Weller DW; Hoh E Nontargeted Biomonitoring of Halogenated Organic Compounds in Two Ecotypes of Bottlenose Dolphins (Tursiops truncatus) from the Southern California Bight. Environ. Sci. Technol 2015, 49 (3), 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Eganhouse RP; Pontolillo J DDE in Sediments of the Palos Verdes Shelf, California: In Situ Transformation Rates and Geo-chemical Fate. Environ. Sci. Technol 2008, 42 (17), 6392–6398. [DOI] [PubMed] [Google Scholar]
- (25).Ferre B; Sherwood C; Wiberg P Sediment transport on the Palos Verdes shelf, California. Cont. Shelf Res 2010, 30, 761–780. [Google Scholar]
- (26).Hose JE; Cross JN; Smith SG; Diehl D Reproductive impairment in a fish inhabiting a contaminated coastal environment off southern California. Environ. Pollut 1989, 57 (2), 139–48. [DOI] [PubMed] [Google Scholar]
- (27).Hoh E; Dodder NG; Lehotay SJ; Pangallo KC; Reddy CM; Maruya KA Nontargeted Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry Method and Software for Inventorying Persistent and Bioaccumulative Contaminants in Marine Environments. Environ. Sci. Technol 2012, 46 (15), 8001–8008. [DOI] [PubMed] [Google Scholar]
- (28).Hoh E; Lehotay SJ; Pangallo KC; Mastovska K; Ngo HL; Reddy CM; Vetter W Simultaneous Quantitation of Multiple Classes of Organohalogen Compounds in Fish Oils with Direct Sample Introduction Comprehensive Two-Dimensional Gas Chromatography and Time-of-Flight Mass Spectrometry. J. Agric. Food Chem 2009, 57 (7), 2653–2660. [DOI] [PubMed] [Google Scholar]
- (29).Myers AL; Watson-Leung T; Jobst KJ; Shen L; Besevic S; Organtini K; Dorman FL; Mabury SA; Reiner EJ Complementary Nontargeted and Targeted Mass Spectrometry Techniques to Determine Bioaccumulation of Halogenated Contaminants in Freshwater Species. Environ. Sci. Technol 2014, 48 (23), 13844–13854. [DOI] [PubMed] [Google Scholar]
- (30).Pangallo K; Nelson RK; Teuten EL; Pedler BE; Reddy CM Expanding the range of halogenated 1′-methyl-1,2′-bipyrroles (MBPs) using GC/ECNI-MS and GC×GC/TOF-MS. Chemosphere 2008, 71 (8), 1557–1565. [DOI] [PubMed] [Google Scholar]
- (31).Pena-Abaurrea M; Jobst KJ; Ruffolo R; Shen L; McCrindle R; Helm PA; Reiner EJ Identification of Potential Novel Bioaccumulative and Persistent Chemicals in Sediments from Ontario (Canada) Using Scripting Approaches with GC×GC-TOF MS Analysis. Environ. Sci. Technol 2014, 48 (16), 9591–9599. [DOI] [PubMed] [Google Scholar]
- (32).Skoczynska E; Korytar P; de Boer J Maximizing Chromatographic Information from Environmental Extracts by GCxGC-ToFMS. Environ. Sci. Technol 2008, 42 (17), 6611–6618. [DOI] [PubMed] [Google Scholar]
- (33).Kumari B; Duhan A Persistence of dicofol residues in cotton lint seed, and soil. Environ. Monit. Assess 2011, 182 (1−4), 129–132. [DOI] [PubMed] [Google Scholar]
- (34).Sanchez AI; Hernando MD; Vaquero JJ; Garcia E; Navas JM Hazard assessment of alternatives to dicofol. J. Environ. Prot 2010, 1 (3), 231–241. [Google Scholar]
- (35).Gillespie MJ; Lythgo CM; Plumb AD; Wilkins JPG A survey comparing the chemical composition of dicofol formulations sold in the UK before and after the introduction of the EC ‘Prohibition Directive 79/117/EEC’. Pestic. Sci 1994, 42 (4), 305–14. [Google Scholar]
- (36).Qiu X; Zhu T; Yao B; Hu J; Hu S Contribution of Dicofol to the Current DDT Pollution in China. Environ. Sci. Technol 2005, 39 (12), 4385–4390. [DOI] [PubMed] [Google Scholar]
- (37).Risebrough RW; Jarman WM; Springer AM; Walker W II; Hunt WG A metabolic derivation of DDE from Kelthane. Environ. Toxicol. Chem 1986, 5 (1), 13–19. [Google Scholar]
- (38).EPA, U. S. Reregistration Eligibility Decision (RED) Dicofol; EPA 738-R-98–018; Prevention, Pesticides, and Toxic Substances (7508C): Washington, DC, USA, November 1998. [Google Scholar]
- (39).Li L; Liu J; Hu J Global Inventory, Long-Range Transport and Environmental Distribution of Dicofol. Environ. Sci. Technol 2015, 49 (1), 212–222. [DOI] [PubMed] [Google Scholar]
- (40).Matthies M; Klasmeier J; Beyer A; Ehling C Assessing Persistence and Long-Range Transport Potential of Current-use Pesticides. Environ. Sci. Technol 2009, 43 (24), 9223–9229. [DOI] [PubMed] [Google Scholar]
- (41).EPA US Estimation Program Interface (EPI) Suite Version 4.10; U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics: Washington, DC, 2011. [Google Scholar]
- (42).Howard PH; Muir DCG Identifying New Persistent and Bioaccumulative Organics Among Chemicals in Commerce. Environ. Sci. Technol 2010, 44 (7), 2277–2285. [DOI] [PubMed] [Google Scholar]
- (43).Howard PH; Muir DCG Identifying New Persistent and Bioaccumulative Organics Among Chemicals in Commerce. III: Byproducts, Impurities, and Transformation Products. Environ. Sci. Technol 2013, 47 (10), 5259–5266. [DOI] [PubMed] [Google Scholar]
- (44).Muir DCG; Howard PH Are There Other Persistent Organic Pollutants? A Challenge for Environmental Chemists. Environ. Sci. Technol 2006, 40 (23), 7157–7166. [DOI] [PubMed] [Google Scholar]
- (45).2012−2013 JWPCP Biennial Receiving Water Monitoring Report; Sanitation Districts of Los Angeles County, Whittier, CA, U.S., 2012–2013. [Google Scholar]
- (46).Schiff K; Gossett R; Ritter K; Tiefenthaler L; Dodder N; Lao W; Maruya K Southern California Bight 2008 Regional Monitoring Program: III. Sediment Chemistry; Southern California Coastal Water Research Project, Costa Mesa, CA, USA, July 2011. [Google Scholar]
- (47).Zeng EY; Tsukada D; Diehl DW; Peng J; Schiff K; Noblet JA; Maruya KA Distribution and Mass Inventory of Total Dichlorodiphenyldichloroethylene in the Water Column of the Southern California Bight. Environ. Sci. Technol 2005, 39 (21), 8170–8176. [DOI] [PubMed] [Google Scholar]
- (48).Falandysz J; Strandberg L; Puzyn T; Gucia M; Rappe C Chlorinated cyclodiene pesticide residues in blue mussel, crab, and fish in the Gulf of Gdansk, Baltic Sea. Environ. Sci. Technol 2001, 35 (21), 4163–4169. [DOI] [PubMed] [Google Scholar]
- (49).Falandysz J; Wyrzykowska B; Warzocha J; Barska I; Garbacik-Wesolowska A; Szefer P Organochlorine pesticides and PCBs in perch Perca fluviatilis from the Odra/Oder river estuary, Baltic Sea. Food Chem 2004, 87 (1), 17–23. [Google Scholar]
- (50).Hoke RA; Ankley GT; Cotter AM; Goldenstein T; Kosian PA; Phipps GL; VanderMeiden FM Evaluation of equilibrium partitioning theory for predicting acute toxicity of field-collected sediments contaminated with DDT, DDE and DDD to the amphipod Hyalella azteca. Environ. Toxicol. Chem 1994, 13 (1), 157–166. [Google Scholar]
- (51).Kazantseva YA; Yarushkin AA; Pustylnyak VO Dichlorodiphenyltrichloroethane technical mixture regulates cell cycle and apoptosis genes through the activation of CAR and ERα in mouse livers. Toxicol. Appl. Pharmacol 2013, 271 (2), 137–143. [DOI] [PubMed] [Google Scholar]
- (52).Lotufo GR; Landrum PF; Gedeon ML Toxicity and bioaccumulation of DDT in freshwater amphipods in exposures to spiked sediments. Environ. Toxicol. Chem 2001, 20 (4), 810–825. [DOI] [PubMed] [Google Scholar]
- (53).Lotufo GR; Landrum PF; Gedeon ML; Tigue EA; Herche LR Comparative toxicity and toxicokinetics of ddt and its major metabolites in freshwater amphipods. Environ. Toxicol. Chem 2000, 19 (2), 368–379. [Google Scholar]
- (54).Nunez G,MA; Estrada I; Calderon-Aranda ES DDT inhibits the functional activation of murine macrophages and decreases resistance to infection by Mycobacterium microti. Toxicology 2002, 174 (3), 201–210. [DOI] [PubMed] [Google Scholar]
- (55).Quensen JF III; Mueller SA; Jain MK; Tiedje JM Reductive dechlorination of DDE to DDMU in marine sediment microcosms. Science (Washington, DC, U. S.) 1998, 280 (5364), 722–724. [DOI] [PubMed] [Google Scholar]
- (56).Fernandez LA; Lao W; Maruya KA; Burgess RM Calculating the Diffusive Flux of Persistent Organic Pollutants between Sediments and the Water Column on the Palos Verdes Shelf Superfund Site Using Polymeric Passive Samplers. Environ. Sci. Technol 2014, 48 (7), 3925–3934. [DOI] [PubMed] [Google Scholar]
- (57).Eganhouse RP; Pontolillo J Old pollutants never die-they just fade away: The fate of DDT on the Palos Verdes Shelf Abstracts of Papers, 234th ACS National Meeting, Boston, MA, United States, August 19−23, 2007. 2007, ENVR-040. [Google Scholar]
- (58).Fernandez LA; Lao W; Maruya KA; White C; Burgess RM Passive Sampling to Measure Baseline Dissolved Persistent Organic Pollutant Concentrations in the Water Column of the Palos Verdes Shelf Superfund Site. Environ. Sci. Technol 2012, 46 (21), 11937–11947. [DOI] [PubMed] [Google Scholar]
- (59).Buser H-R DDT, A Potential Source of Environmental Tris(4-chlorophenyl)methane and Tris(4-chlorophenyl)methanol. Environ. Sci. Technol 1995, 29 (8), 2133–9. [DOI] [PubMed] [Google Scholar]
- (60).Jarman WM; Simon M; Norstrom RJ; Burns SA; Bacon CA; Simoneit BRT; Risebrough RW Global distribution of tris(4-chlorophenyl)methanol in high tropic level birds and mammals. Environ. Sci. Technol 1992, 26 (9), 1770–4. [Google Scholar]
- (61).Walker W II; Risebrough RW; Jarman WM; De Lappe BW; Tefft JA; DeLong RL Identification of tris(chlorophenyl)-methanol in blubber of harbor seals from Puget Sound. Chemosphere 1989, 18 (9–10), 1799–804. [Google Scholar]
- (62).Buser HR; Zook DR; Rappe C Determination of methyl sulfone-substituted polychlorobiphenyls by mass spectrometric techniques with application to environmental samples. Anal. Chem 1992, 64 (10), 1176–83. [Google Scholar]
- (63).Lebeuf M; Bernt KE; Trottier S; Noel M; Hammill MO; Measures L Tris (4-chlorophenyl) methane and tris (4-chlorophenyl) methanol in marine mammals from the Estuary and Gulf of St. Lawrence. Environ. Pollut 2001, 111 (1), 29–43. [DOI] [PubMed] [Google Scholar]
- (64).Holmstrand H; Zencak Z; Mandalakis M; Andersson P; Gustafsson O Chlorine isotope evidence for the anthropogenic origin of tris-(4-chlorophenyl)methane. Appl. Geochem 2010, 25 (9), 1301–1306. [Google Scholar]
- (65).Blinn RC; Gaston LK Composition analysis of technical-grade DDT. SRI (Stanford Res. Inst.), Pesticide Res. Bull 1963, 3 (1), 1, 3–4. [Google Scholar]
- (66).Haller HL; Bartlett PD; Drake NL; Newman MS; Cristol SJ; Eaker CM; Hayes RA; Kilmer GW; Magerlein B; Mueller GP; Schneider A; Wheatley W Chemical composition of technical DDT. J. Am. Chem. Soc 1945, 67, 1591–602. [Google Scholar]
- (67).Council Directive 79/1117/EEC of 21 December 1978 prohibiting the placing on the market and use of plant protection products containing certain active substances.
- (68).Munoz-Arnanz J; Jimenez B New DDT inputs after 30 years of prohibition in Spain. A case study in agricultural soils from southwestern Spain. Environ. Pollut. (Oxford, U. K.) 2011, 159 (12), 3640–3646. [DOI] [PubMed] [Google Scholar]
- (69).Tao S; Li BG; He XC; Liu WX; Shi Z Spatial and temporal variations and possible sources of dichlorodiphenyltrichloroethane (DDT) and its metabolites in rivers in Tianjin, China. Chemosphere 2007, 68 (1), 10–16. [DOI] [PubMed] [Google Scholar]
- (70).EURL-SRM, Analysis of dicofol via QuECHERS-use of isotope labeled dicofol to improve precision EU Reference Laboratory for Pesticides Requiring Single Residue Methods; CVUA Stuttgart, Germany, 2013. [Google Scholar]
- (71).Kannan K; Kajiwara N; Le Boeuf BJ; Tanabe S Organochlorine pesticides and polychlorinated biphenyls in California sea lions. Environ. Pollut. (Oxford, U. K.) 2004, 131 (3), 425–434. [DOI] [PubMed] [Google Scholar]
- (72).Boethling RS; Howard PH; Meylan W; Stiteler W; Beauman J; Tirado N Group contribution method for predicting probability and rate of aerobic biodegradation. Environ. Sci. Technol 1994, 28 (3), 459–65. [DOI] [PubMed] [Google Scholar]
- (73).Boethling RS; Lynch DG; Thom GC Predicting ready biodegradability of premanufacture notice chemicals. Environ. Toxicol. Chem 2003, 22 (4), 837–844. [PubMed] [Google Scholar]
- (74).Boethling RS; Sabljic A Screening-level model for aerobic biodegradability based on a survey of expert knowledge. Environ. Sci. Technol 1989, 23 (6), 672–9. [Google Scholar]
- (75).Howard PH; Boethling RS; Stiteler WM; Meylan WM; Hueber AE; Beauman JA; Larosche ME Predictive model for aerobic biodegradability developed from a file of evaluated biodegradation data. Environ. Toxicol. Chem 1992, 11 (5), 593–603. [Google Scholar]
- (76).Tunkel J; Howard PH; Boethling RS; Stiteler W; Loonen H Predicting ready biodegradability in the Japanese Ministry of International Trade and Industry Test. Environ. Toxicol. Chem 2000, 19 (10), 2478–2485. [Google Scholar]
- (77).Santos-Neto EB; Azevedo-Silva CE; Bisi TL; Santos J; Meirelles ACO; Carvalho VL; Azevedo AF; Guimarães JE; Lailson-Brito J Organochlorine concentrations (PCBs, DDTs, HCHs, HCB and MIREX) in delphinids stranded at the northeastern Brazil. Sci. Total Environ 2014, 472, 194–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.