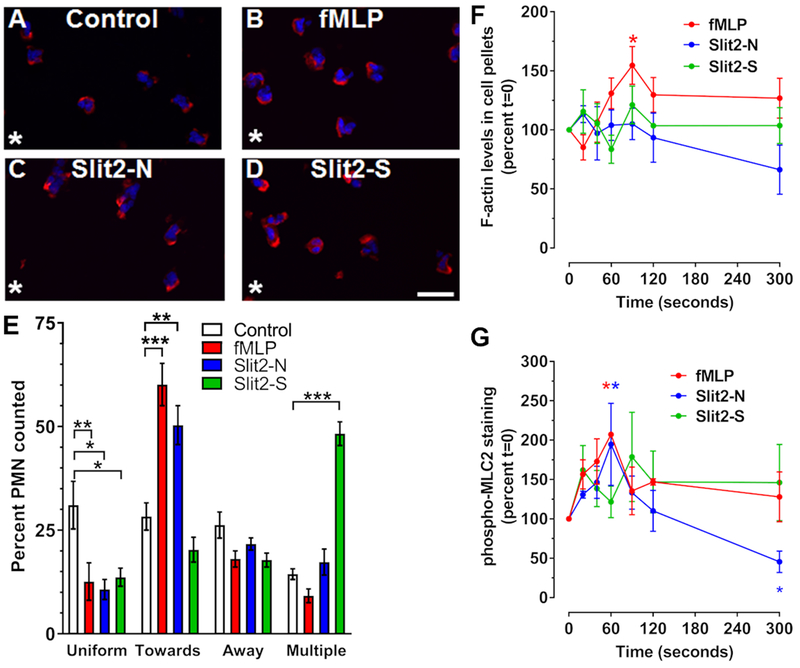
Figure 3. Only Slit2-N and fMLP generate polarized F-actin.
Neutrophils were incubated in the presence or absence of a single point source (asterisk) of (A) buffer control, (B) fMLP, (C) Slit2-N, or (D) Slit2-S for 10 minutes. Cells were then fixed and stained for F-actin with phalloidin-Alexa 555 (red) and counterstained with DAPI (blue). Images are from one of 3 different donors. Bar is 20 μm. (E) Quantification of phalloidin staining location, indicating the percent of cells with F-actin at the edge of the cell either towards or away from the stimulus, or cells showing multipolar, or uniform staining. (F-G) Neutrophils were incubated with fMLP, Slit2-N, or Slit2-S, for 20, 40, 60, 90, 120, and 300 seconds. Cells were then lysed in Triton-X 100 buffer to isolate cytoskeletal and cytoplasmic proteins. (F) Triton-X 100 insoluble cytoskeletal proteins were analyzed by PAGE and stained with Coomassie to quantify F-actin. (G) Cytoskeletal proteins were also analyzed for phosphorylated myosin light chain 2 (pMLC2) by western blotting. All values are mean ± SEM for neutrophils from 3-4 different donors. * indicates p < 0.05, ** p < 0.01, *** p < 0.001 compared to the t=0 buffer control (1-way ANOVA, Dunnett’s test).