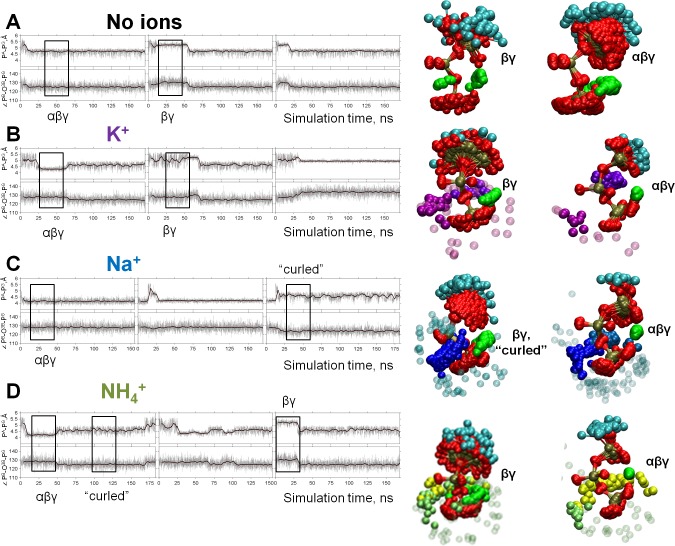
Figure 4. Dynamics of the phosphate chain of the Mg-ATP complex with and without monovalent cations.
Each left panel shows the PA-PG distance (upper trace) and the PB-O3B-PG angle (bottom trace) in the course of MD simulations. Thin gray lines show actual values measured from each frame of the MD simulation, the bold black lines show moving average with a 2-ps window. Black boxes indicate fragments of simulations chosen for the analyses of particular types of interaction between the Mg2+ ion and the triphosphate chain; the respective conformations of Mg-ATP are shown on the right. The analysis was performed as shown in Figure 2B. The color scheme is as in Figure 1. (A) no added ions; (B–D) MD simulations in the presence of K+, Na+, and NH4+, respectively.
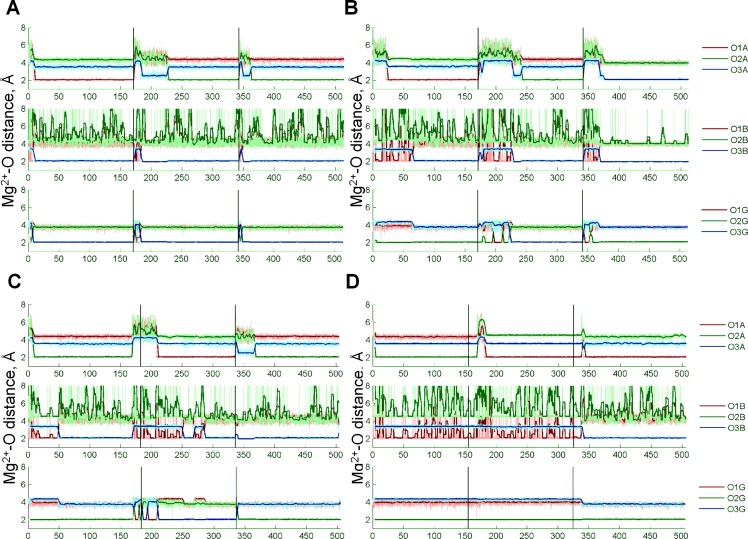
Figure 4—figure supplement 1. Coordination of the Mg22+ion by the oxygen atoms of the ATP phosphate chain during MD simulations.
Figure 4—figure supplement 2. Estimation of correlation times for the PA-PG distances.
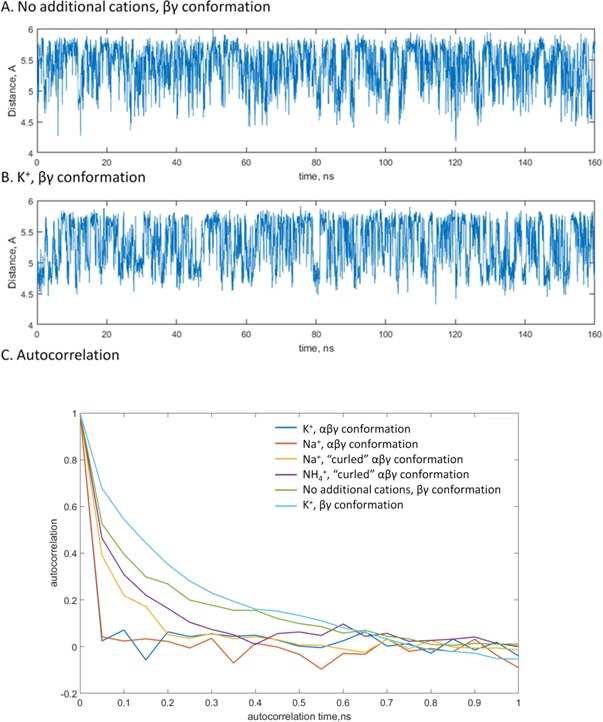
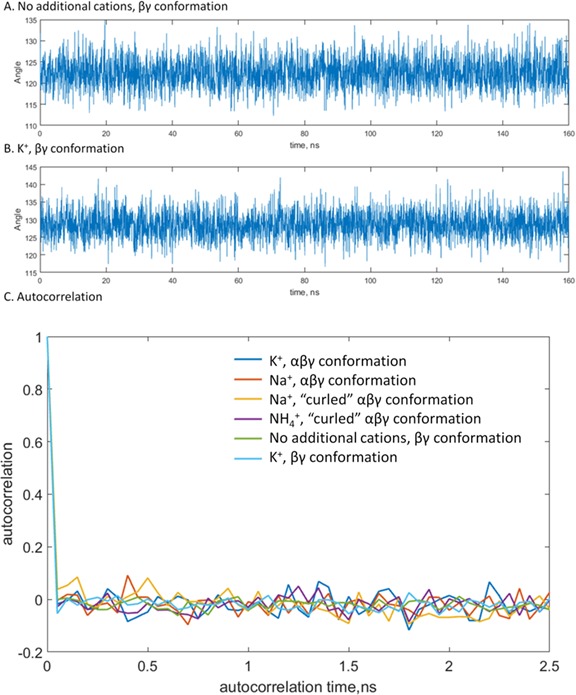
Figure 4—figure supplement 3. Estimation of correlation times for the PB-O-PG angles.