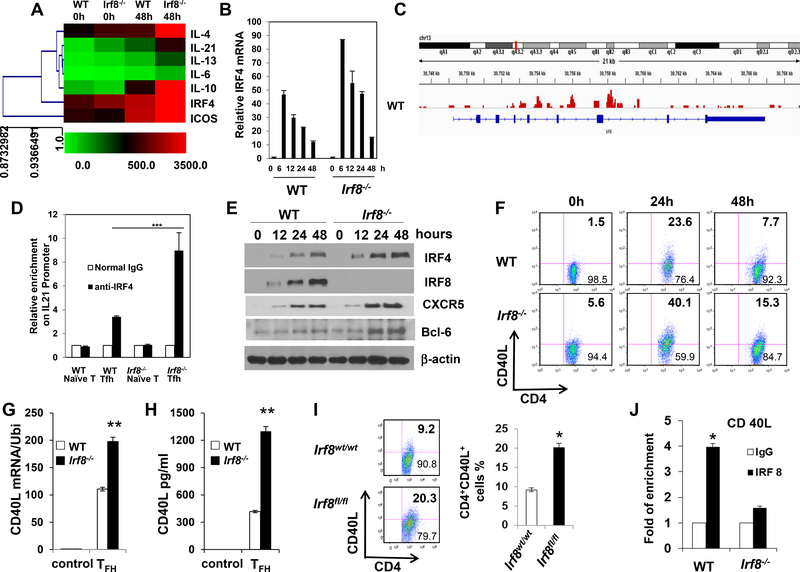
Figure 7. Regulation of IRF4 expression by IRF8.
(A) Purified CD4+ T cells from WT or Irf8−/− mice were stimulated with or without anti-CD3 and anti-CD28 for 48h in the presence of IL-21. Microarray analysis of the gene expression by WT and Irf8‒/‒ CD4+ T cells under TFH condition. (B) Quantitative real-time RT-PCR analysis of irf4 gene level in WT and Irf8‒/‒ CD4+ T cells under TFH condition for time course (n = 5). The ubiquitin gene (Ubi) was used as an internal control. (C) IRF8 ChIP-seq signals at the IRF4 gene locus. Blue line denotes the range of the IRF4 gene locus, and the peak patterns in red represent the ChIP-seq signals of IRF8 binding. Signals represent DNA fragments that were captured by the IRF8 antibody through ChIP. (D) Purified CD4+ T cells from WT or Irf8−/− mice were stimulated with anti-CD3 and anti-CD28 for 48h in the presence of IL-21, followed by ChIP assay. Three micrograms of an anti-IRF4 antibody or isotype-matched IgG as control antibody were used in the immunoprecipitation step. PCR was used to quantify the amount of precipitated DNA with primers flanking the irf4-binding site of the IL-21 promoter region. Each bar represents mean ± S.D. from three independent experiments, unpaired Student’s t-test, *P<0.05, versus WT cells. (E) Western blot analysis of IRF4, IRF8, Bcl-6 and CXCR5 protein levels in WT and Irf8‒/‒ CD4+ T cells under TFH condition for time course. The β-actin was used as an internal control. (F) Purified CD4+ T cells from WT or Irf8‒/‒ mice were stimulated with anti-CD3 and anti-CD28 antibody for 48h. CD40L expression was analyzed by flow cytometry gated on CD4+ T cells and used isotype control. (G) Cd40lg RNA level was analyzed by real-time RT-PCR (n = 5). (H) Soluble CD40L protein level in culture media was measured by ELISA. (I) The experiment was repeated by using purified CD4+ T cells of Lck-Cre+Irf8wt/wt (n = 6) or Lck-Cre+Irf8fl/fl (n = 6) mice. (J) ChIP assay of CD4+ T cells from WT and Irf8‒/‒ mice was analyzed for binding of IRF8 to the Cd40lg promoter (n = 4). Results shown are representative of three independent experiments. Data are given as means ± SEM.