Abstract
Objective
The study aimed to explore the relationship of the telomere length with type 2 diabetes mellitus (DM) among patients with ischemic heart disease (IHD).
Method
This 2-year cross-sectional study included 130 male patients diagnosed with IHD through echocardiography and coronary angiography, wherein consecutive IHD patients with type 2 DM (65) and without type 2 DM (65) were selected. Baseline characteristics including age, gender, body mass index, and blood pressure were recorded. Laboratory investigations such as random blood sugar (RBS), fasting lipid profile, serum creatinine, and serum urea levels were measured. Quantitative real-time polymerase chain reaction was used for the measurement of the telomere length. The logistic regression analysis was used to predict the relationship of the telomere length with age and type 2 DM among patients with IHD.
Results
All the patients in the study were men, and most of them (diabetics = 22; nondiabetics = 20) were aged between 56 and 65 years. Age (p = 0.003), telomere length (p < 0.001), RBS (p < 0.001), serum creatinine (p < 0013), and serum urea (p < 0.04) were significantly higher in the diabetic subset than in the nondiabetic subset. No significant relationship was observed between age and the telomere length (p = 0.813); however, the mean telomere length was significantly high among the patients with type 2 DM than those without type 2 DM (p = 0.005). The logistic regression analysis showed that the telomere shortening (p = 0.00019) and RBS (p < 0.0001) were the significant risk factors for type 2 DM in patients with IHD.
Conclusion
The telomere shortening was significantly correlated with type 2 DM among the patients with IHD. However, multicentric studies with larger samples are required to validate the current observation.
Keywords: Diabetes, Ischemic heart disease, qRT-PCR, Telomere, Telomere length
1. Introduction
Worldwide, ischemic heart disease (IHD) is the major cause of death and acquired disability. Globally, it accounts for approximately 8.1 million deaths every year.1 Along with traditional risk factors such as systemic hypertension, dyslipidemia, and body mass index (BMI), hyperglycemia is also an independent risk factor for the development of IHD.2 Chronic hyperglycemia in patients with type 2 diabetes mellitus (DM) increases the oxidative stress, interferes with telomerase function, and results in telomere attrition or shortening.3 Furthermore, it was reported that the telomere length was shorter in the diabetic patients than in the healthy individuals.4
Telomeres, found at the end of chromosomes, shorten with each cell cycle and reflect the aging of the organisms. Therefore, the telomere length is identified as the biological marker for cellular aging.5 Male gender, smoking, adiposity, inflammation, obesity, physical inactivity, insulin resistance, oxidative stress, and ultraviolet irradiation along with advancing age are the attributed risk factors for the shortening of telomeres.5, 6, 7 Multiple techniques have emerged to determine the telomere length8; however, quantitative polymerase chain reaction (qPCR) is considered as an amenable technique even in the analysis of small amounts of the DNA sample.9 The qPCR technique expresses the telomere DNA content as the ratio of telomeric product (T)/single-copy gene (S) product (T/S ratio).10
Shortening of the telomere is related to an increased risk of age-related diseases such as peripheral vascular disease, IHD, myocardial infarction (MI), and cancer.5 Shortening of telomeres is independently associated with the progression of metabolic syndromes via affecting the cellular metabolic rate.11, 12 Furthermore, some studies have reported a heritable shorter telomere as a risk factor in the development of type 2 DM.13, 14 The telomere shortening results in premature beta (β)-cell senescence, reduced β-cell mass, impaired insulin secretion, and glucose intolerance.3 Several studies have reported association between the telomere length and essential hypertension, obesity, vascular dementia, and mortality due to heart disease.15 However, there is a scarcity of literature exploring the relationship between the telomere length and type 2 DM in patients with IHD. Hence, this interesting observation compelled us to explore the relationship of the telomere length with type 2 DM among patients with IHD.
2. Methods
2.1. Study design
This 1-year cross-sectional study was conducted at the Department of General Medicine at Jawaharlal Nehru Medical College, KAHER. The ethical approval was obtained from Institutional Ethical and Research Committee, and the trial was registered under Clinical Trials Registry of India no. CTRI/2018/02/011663. Informed written consent was also obtained from the patients before their participation in the study. A total of 130 male patients with IHD, diagnosed through echocardiography and coronary angiography, were included in the study, wherein consecutive IHD patients with type 2 DM (65) and without type 2 DM (65) were selected. While female patients, patients with history of smoking, chronic alcoholism, and systemic illnesses were excluded from the study.
2.2. Data collection
Demographic data including age and gender of the patients were noted. Height and weight of the patients were measured for the calculation of BMI using a standard protocol.16 Blood samples (5 ml) drawn from an antecubital vein were stored in a vacutainer containing a small amount of ethylenediaminetetraacetic acid (EDTA). Laboratory investigations such as random blood sugar (RBS), fasting lipid profile, serum creatinine, and serum urea levels of the patients were measured. Blood pressure (BP) was recorded using a sphygmomanometer. The ejection fraction was assessed using echocardiography.
2.3. DNA extraction, amplification, and measurement
DNA was extracted from the peripheral blood leukocytes using Qiagen DNA Mini Kit (QIAGEN Inc., California, United States). The telomere length of the isolated DNA was measured using quantitative real-time PCR (RT-PCR) (Applied Biosystems, California, United States) as T/S ratio.17 Cycling conditions for both the telomere and 36B4 amplicons are as follows: 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min, followed by a melt curve with little modifications as described previously.17 The 36B4 is a single-copy gene and serves as a reference gene in the conventional qPCR technique in the measurement of telomeres. All samples were analyzed in the ABI StepOnePlus RT-PCR System with SDS version StepOnePlus software (Applied Biosystems, California, United States).
2.4. Statistical analysis
Data were entered and coded in Microsoft excel spreadsheet. R-3.4.1 was used to analyze the data. Categorical data were represented in the numeric form, and continuous data were presented as mean ± standard deviation. Independent t-test was used to compare the mean difference between the two groups. Multivariate regression analysis was performed to determine the variables affecting the telomere length. Logistic regression analysis was used to predict the relationship of the telomere length, age, BMI, RBS, serum urea, and serum creatinine with the diabetic status among patients with IHD.
3. Results
A total of 130 IHD patients with or without type 2 DM were assessed during the study period. Most of the patients in both the diabetic (22) and nondiabetic (20) subsets were aged between 56 and 65 years. However, in the diabetic subset, many patients (61) were aged > 46 years (Table 1). All the patients in both the subsets were men; owing to the direct influence of female gender on the telomere length, women were excluded from our study.
Table 1.
Age distribution of diabetic and nondiabetic patients with ischemic heart disease.
| Age (years) | Ischemic heart disease |
|
|---|---|---|
| Diabetic (n = 65) | Nondiabetic (n = 65) | |
| 25–35 | 0 | 4 |
| 36–45 | 4 | 16 |
| 46–55 | 18 | 10 |
| 56–65 | 22 | 20 |
| > 65 | 21 | 15 |
Independent t-test was used to compare the baseline characteristics in the diabetic and nondiabetic subsets (Table 2). IHD patients with type 2 DM were older, had shorter telomere length (p < 0.001), and had higher BMI, systolic and diastolic BP, serum creatinine, serum urea, and triglyceride levels, whereas total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were low compared with nondiabetic patients. Raised enzyme levels and pain, two-vessel coronary artery disease and drug history (aspirin + clopidogrel + atorvastatin + metoprolol + pantocid) were high in patients with type 2 diabetes. None of the diabetic patients had prior history of MI and coronary artery revascularization. However, a significant difference was observed between the diabetic and nondiabetic subset in terms of age (p = 0.003), telomere length (p < 0.001), RBS level (p < 0.001), serum creatinine level (p < 0.013), and serum urea level (p < 0.04).
Table 2.
Baseline characteristics of diabetic and nondiabetic patients with ischemic heart disease.
| Variable | Diabetic | Nondiabetic | p value |
|---|---|---|---|
| Age (years) | 61.26 ± 10.44 | 54.92 ± 13.04 | 0.003∗ |
| Telomere length | 1.32 ± 0.36 | 2.00 ± 0.52 | <0.001∗ |
| BMI (kg/m2) | 24.64 ± 2.52 | 23.66 ± 3.19 | 0.054 |
| RBS (mg/dL) | 229 ± 71.09 | 136.29 ± 50.25 | <0.001∗ |
| Blood pressure (mm Hg) | |||
| Systolic | 122.68 ± 21.74 | 122.43 ± 18.83 | 0.945 |
| Diastolic | 79.2 ± 10.39 | 78.37 ± 11.98 | 0.674 |
| Renal functions(mg/dL) | |||
| Serum creatinine | 1.12 ± 0.54 | 0.93 ± 0.28 | 0.013∗ |
| Serum urea | 38.09 ± 27.83 | 29.72 ± 16.88 | 0.04∗ |
| Lipids profile (mg/dL) | |||
| Total cholesterol | 179.08 ± 28.12 | 181.77 ± 38.42 | 0.649 |
| LDL cholesterol | 123.34 ± 25.02 | 127.32 ± 31.42 | 0.425 |
| HDL cholesterol | 37.74 ± 7.12 | 39.02 ± 7.02 | 0.305 |
| Triglycerides | 135.72 ± 28.12 | 126.77 ± 41.13 | 0.150 |
| Signs and symptoms | |||
| Elevated enzyme | 13(20) | 13(20) | 0.6128 |
| Pain | 32(49.23) | 27(41.54) | |
| Both | 20(30.77) | 25(38.46) | |
| Prior myocardial infarction | |||
| Absent | 65(100) | 63(96.92) | 0.4961 |
| Present | 0(0) | 2(3.08) | |
| Coronary artery vessel involved | |||
| 1 vessel | 1(1.54) | 2(3.08) | 0.4066 |
| 2 vessels | 34(52.31) | 26(40) | |
| 3 vessels | 30(46.15) | 37(56.92) | |
| Drug history | |||
| 1,3,4,5,6 | 11(16.92) | 10(15.38) | 0.4167 |
| 1,2,3,6 | 5(7.69) | 10(15.38) | |
| 1,2,3,4,5,6 | 41(63.08) | 34(52.31) | |
| 1,2,4,5,6 | 8(12.31) | 11(16.92) | |
BMI, body mass index; RBS, random blood sugar; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SD, standard deviation; 1, aspirin; 2, clopidogrel, 3, atorvastatin; 4, metoprolol; 5, pantocid.
Data were expressed in mean ± SD and n (%).
∗Significant.
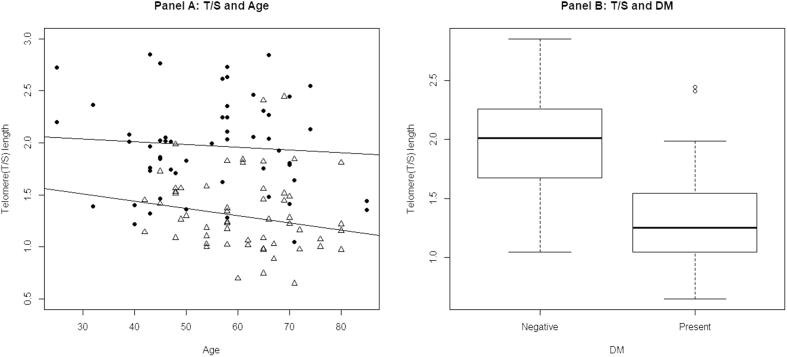
Relationship of the telomere length with chronological age among IHD patients with and without diabetes is shown in Fig. 1 (panel A). Both in diabetic and nondiabetic patients, as the age increases, the telomere length slowly decreased. However, the decrease in the telomere length in nondiabetic patients was less compared with diabetic patients (p = 0.813). While comparing the diabetic and nondiabetic subset with the telomere length, the telomere length was significantly higher in the diabetic subset than in the nondiabetic subset (p = 0.005; Panel B, Fig. 1).
Fig. 1.
Relationship of age and the diabetic status with the telomere length. Panel A: relationship between age and the telomere length; as the age increases, the telomere length slowly decreases in diabetic patients (lower line) and nondiabetic patients (upper line). Panel B: relationship between type 2 diabetes mellitus and the telomere length; the telomere length was high in the nondiabetic (absent) subset, whereas it was low in the diabetic (present) group. DM, diabetes mellitus; T/S, telomeric product/single-copy gene product.
The risk factors influencing the telomere length were shown in Table 3. Of all, the diabetes status was found to be significantly correlated with the telomere length (p < 0.0001). From the logistic regression analysis, odds ratio showed that the telomere shortening (p = 0.00019), RBS (p < 0.0001) and serum urea were the significant risk factors for type 2 DM in patients with IHD. However, comparatively, the telomere shortening was 4.3 times higher in patients with type 2 DM (Table 4).
Table 3.
Multivariate regression analysis for the variables influencing the telomere length.
| Variables | Estimate (95% CI) | P-value |
|---|---|---|
| Age | −0.0014 (−0.0053–0.0028) | 0.5302 |
| Diabetic patients | −0.4528 (−0.5640–−0.3000) | <0.0001 |
| Dyslipidemia positive | −0.1014 (−0.2563–0.0452) | 0.2027 |
| BP | −0.0003 (−0.0033–0.0032) | 0.8748 |
| BMI | 0.0001 (−0.0160–0.0191) | 0.9880 |
| RBS | 0.0001 (−0.0009–0.0007) | 0.9473 |
| Serum urea | 0.0008 (−0.0020–0.0036) | 0.5652 |
| Serum creatinine | 0.0344 (−0.1081–0.1583) | 0.6215 |
| Sign (pain) | −0.0617 (−0.1701–0.0543) | 0.3068 |
| Sign (both enzymes elevated, pain) | −0.1095 (−0.2487–0.0052) | 0.1157 |
| Prior myocardial infarction present | −0.2176 (−0.5960–0.1082) | 0.2194 |
| coronary artery, two vessels involved | −0.1922 (−0.3917–0.0490) | 0.1016 |
| coronary artery, three vessels involved | −0.1894 (−0.3900–0.0515) | 0.1079 |
| Drug history | −0.0725 (−0.2212–0.3234) | 0.3169 |
CI, confidence interval; BP, blood pressure; BMI, body mass index; RBS, random blood sugar.
Table 4.
Logistic regression for the diabetic and nondiabetic groups.
| Estimate | Odds ratio (95% CI) | p-value | |
|---|---|---|---|
| Diabetic | |||
| Age | 0.05 | 1.0607 (0.9956–1.1413) | 0.0821 |
| Telomere length | −4.343 | 0.0139 (0.0013–0.0854) | 0.00019∗ |
| BMI | 0.10 | 1.0427 (1.0255–1.0673) | 0.45 |
| RBS | 0.046 | 0.9576 (0.9144–0.9981) | <0.0001∗ |
| Serum urea | −0.057 | 3.3682 (0.5078–35.1792) | 0.0205∗ |
| Serum creatinine | 0.867 | 1.0756 (0.8434–1.3977) | 0.3799 |
CI, confidence interval; BMI, body mass index; RBS, random blood sugar.
∗Significant.
4. Discussion
The telomere shortening is hypothesized as the biomarker for age and age-related diseases.18 Diabetes, complicated by cardiovascular diseases, increases mortality and results in higher levels of oxidative stress and inflammation, which in turn accelerate the telomere shortening.11 Similarly, in our study, the telomere length in IHD patients with type 2 DM was significantly low compared with IHD patients without type 2 DM. Based on these findings, we can hypothesize that shorter telomeres promote the onset of type 2 DM in patients with IHD. Hence, the measurement of the telomere length may signify prognostic information for clinicians in the management of IHD patients with type 2 DM. Similarly, several studies19, 20 in different regions demonstrated that shorter telomeres, independently and significantly, predict the increased risk of type 2 DM. A literature-based meta-analysis also reported that the telomere shortening is an independent risk factor associated in the incidence of type 2 DM.21 In contrast, a study by Aviv et al22 reported that the telomere shortening in patients with type 2 DM could potentially be explained by the genetic regulation.
The telomere shortening indicates an important molecular mechanism for biological/vascular aging.23 Although, in our study, increased age influenced the telomere length, it was insignificant. In contrast, studies conducted by Masi et al24 (r = −0.150; p = 0.002) and Eguchi et al25 (r = −0.194; p < 0.001) reported a negative correlation between the telomere length and age. Men have shorter telomere length owing to the higher prevalence of degenerative diseases compared with women.26 Therefore, female patients were excluded from the study. However, there is a lack of literature to comment these findings. A similar study conducted by Tentolouris et al23 in men reported that the telomere length in type 2 DM patients with microalbuminuria was shorter (6.64 ± 0.74 vs. 7.23 ± 1.01 kb, respectively, p = 0.004) than those without microalbuminuria.
In our study, the RBS, serum creatinine, and urea levels were significantly higher in IHD patients with type 2 DM. However, serum creatinine and urea levels were not found significant in IHD patients with type 2 DM. A study conducted by Gurung et al27 reported that increased oxidative stress leads to telomere shortening and predicts the renal impairment in patients with type 2 DM.
Overall, the present study implicates that IHD patients with type 2 DM have low telomere length when compared with IHD patients without type 2 DM; the influence of age on the telomere length was statistically significant. The present study has few potential limitations such as the small sample size enrolled in the study, which is a major limitation. Owing to the cross-sectional design of the study, large prospective multicentric studies are required to validate the exact relationship involved in the shortening of the telomere length in type 2 DM patients with IHD. However, this is the first study of its kind reporting a relationship between the telomere length and type 2 DM in patients with IHD.
Overall, the telomere shortening was significantly correlated with type 2 DM among patients with IHD. However, multicentric studies with larger samples are required to validate the current observations. Moreover, this study will be a great reflection of upcoming genetics in Indian population.
What is already known?
Shortening of telomere is associated with an increased risk of age-related diseases such as peripheral vascular disease, IHD, MI, and cancer. Previous studies have evidenced a heritable shorter telomere as a risk factor in the development of type 2 DM.
What this study adds?
The present study explored the relationship between the telomere length and type 2 DM among patients with IHD.
Source of funding
Nil.
Conflict of interest
All authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2018.09.007.
Contributor Information
Shobhit Piplani, Email: shobhitpiplani@aol.com.
Nadezdha Niyarah Alemao, Email: niyarahalemao99@ymail.com.
Madhav Prabhu, Email: madhavprabhukle@gmail.com.
Sameer Ambar, Email: drsameerambar@rediffmail.com.
Yashasvi Chugh, Email: yashasvichugh@hotmail.com.
Sanjay Kumar Chugh, Email: skchughcardiology@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shepard D., VanderZanden A., Moran A., Naghavi M., Murray C., Roth G. Ischemic heart disease worldwide, 1990 to 2013: estimates from the global burden of disease study 2013. Circ Cardiovasc Qual Outcome. 2015;8:455–456. doi: 10.1161/CIRCOUTCOMES.115.002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dresslerova I., Vojacek J. Diabetes mellitus and ischemic heart disease. Vnitr Lek. 2010;56:301–306. [PubMed] [Google Scholar]
- 3.Elks C.E., Scott R.A. The long and short of telomere length and diabetes. Diabetes. 2014;63:65–67. doi: 10.2337/db13-1469. [DOI] [PubMed] [Google Scholar]
- 4.Testa R., Olivieri F., Sirolla C. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med. 2011;28:1388–1394. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- 5.Weischer M., Bojesen S.E., Cawthon R.M., Freiberg J.J., Tybjærg-Hansen A., Nordestgaard B.G. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32:822–829. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- 6.Butt H., Atturu G., London N., Sayers R., Bown M. Telomere length dynamics in vascular disease: a review. Eur J Vasc Surg. 2010;40:17–26. doi: 10.1016/j.ejvs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Han J., Qureshi A.A., Prescott J. A prospective study of telomere length and the risk of skin cancer. J Investig Dermatol. 2009;129:415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montpetit A.J., Alhareeri A.A., Montpetit M. Telomere length: a review of methods for measurement. Nurs Res. 2014;63:289. doi: 10.1097/NNR.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Callaghan N.J., Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aviv A., Hunt S.C., Lin J., Cao X., Kimura M., Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134–e. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Ning Z., Lee Y., Hambly B.D., McLachlan C.S. Shortened leukocyte telomere length in type 2 diabetes mellitus: genetic polymorphisms in mitochondrial uncoupling proteins and telomeric pathways. Clin Transl Med. 2016;5:8. doi: 10.1186/s40169-016-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi Y., Maeda T., Guan J.-Z., Oyama J., Sugano M., Makino N. Diagonal earlobe crease are associated with shorter telomere in male Japanese patients with metabolic syndrome. Circ J. 2009;73:274–279. doi: 10.1253/circj.cj-08-0267. [DOI] [PubMed] [Google Scholar]
- 13.Nai-chieh Y., Chen B.H., Song Y. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes. 2012;61:2998–3004. doi: 10.2337/db12-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q., Zhao X., Yu L. Association of leukocyte telomere length with type 2 diabetes in mainland Chinese populations. J Clin Endocrinol Metab. 2012;97:1371–1374. doi: 10.1210/jc.2011-1562. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick A.L., Kronmal R.A., Gardner J.P. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2006;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 16.Statistics NCfH. NCHS; Hyattsville, MD: 2013. Anthropometry Procedures Manual—National Health and Nutrition Examination Survey (NHANES) [Google Scholar]
- 17.Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47–e. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'mello M.J., Ross S.A., Briel M., Anand S.S., Gerstein H., Paré G. The association between shortened leukocyte telomere length and cardio-metabolic outcomes: a systematic review and meta-analysis. Circ Cardiovasc Genet. 2014;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Dong X., Cao L. Association between telomere length and diabetes mellitus: a meta-analysis. J Int Med Res. 2016;44:1156–1173. doi: 10.1177/0300060516667132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Zhu Y., Lin J. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes. 2014;63:354–362. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willeit P., Raschenberger J., Heydon E.E. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS One. 2014;9:e112483. doi: 10.1371/journal.pone.0112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aviv A., Kark J.D., Susser E. Telomeres, atherosclerosis, and human longevity: a causal hypothesis. Epidemiology. 2015;26:295. doi: 10.1097/EDE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tentolouris N., Nzietchueng R., Cattan V. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30(11):2909–2915. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- 24.Masi S., D'Aiuto F., Cooper J. Telomere length, antioxidant status and incidence of ischaemic heart disease in type 2 diabetes. Int J Cardiol. 2016;216:159–164. doi: 10.1016/j.ijcard.2016.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi K., Honig L.S., Lee J.H., Hoshide S., Kario K. Short telomere length is associated with renal impairment in Japanese subjects with cardiovascular risk. PLoS One. 2017;12:e0176138. doi: 10.1371/journal.pone.0176138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett E.L., Richardson D.S. Sex differences in telomeres and lifespan. Aging Cell. 2011;10:913–921. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 27.Gurung R.L., Yiamunaa M., Liu S., Liu J.-J., Lim S.C. Short leukocyte telomere length predicts albuminuria progression in individuals with type 2 diabetes. Kidney Int Rep. 2017;3(3):592–601. doi: 10.1016/j.ekir.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.