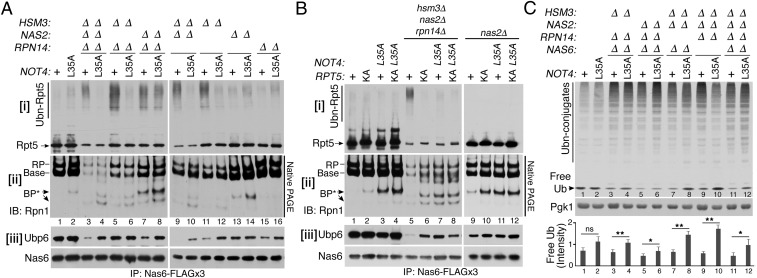
Fig. 4.
Not4-mediated ubiquitination of Rpt5 blocks recruitment of Ubp6 and Rpn1 during endogenous base assembly. (A and B) Not4-mediated ubiquitination of Rpt5 blocks incorporation of Rpn1 and Ubp6 into the base. Assembly intermediates were isolated using 3× FLAG-tagged Nas6 and were analyzed by 4–12% Bis-Tris SDS/PAGE and immunoblotting for indicated proteins in i and iii (See SI Appendix, Supplementary Materials and Methods for details). Rpn1-containing species were detected by 3.5% native PAGE and immunoblotting (ii). Nas6, a loading control (iii). (C) Not4-mediated control of proteasome assembly regulates the free ubiquitin pool. Whole-cell lysates (20 μg) were subjected to 10% Bis-Tris SDS/PAGE and immunoblotted for ubiquitin (SI Appendix, Supplementary Materials and Methods). Pgk1, loading control. Relative signal intensities of free ubiquitin bands were quantified (n = 4; mean ± SEM; ns, not significant; *P < 0.05; **P < 0.01).