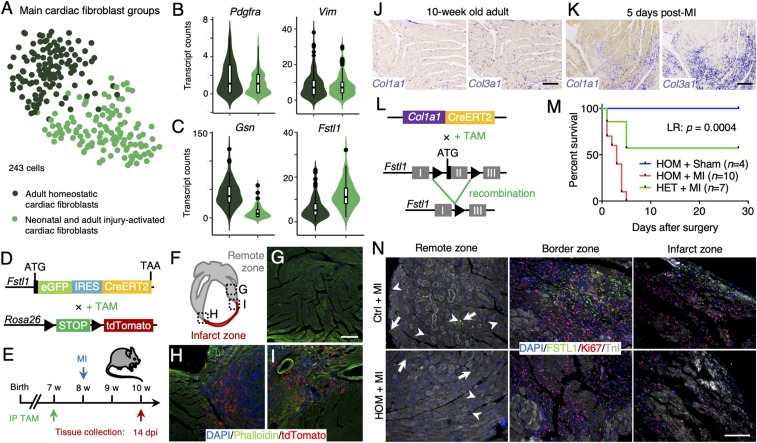
Fig. 7.
FSTL1 expression is characteristic to a population of injury-activated fibroblasts and is crucial in preventing cardiac rupture upon damage. (A) t-SNE map of cardiac fibroblast subclusters identified using the RaceID2 algorithm projecting the different experimental conditions. (B and C) Violin plots showing the expression of Pdgfra, Vim (B), and Gsn and Fstl1 (C) in neonatal and injury-activated fibroblasts (light green) and homoeostatic adult fibroblasts (dark green). (D) Schematic representation of the generation of mice expressing eGFP and CreERT2 by cassette insertion within the Fstl1 protein coding region, enabling lineage tracing by crossing it with LSL-tdTomato mice. (E) Experimental timeline. (F–I) Progeny (red) of Fstl1-expressing fibroblasts is enriched in the scar tissue 14 dpi (H and I), while tdTomato labeling in the remote area remains low (G). Nuclei were counterstained with DAPI (blue) and F-actin visualized by phalloidin (green). (J and K) In situ hybridization of Col1a1 and Col3a1 in adult homoeostatic heart (J) and 5 d after MI (K). (L) Schematic representation of the generation of conditional knockout (cKO) mice by crossing Col1a1-CreERT2 mice with Fstl1flox/flox mice, resulting in the depletion of Fstl1 exon 2 upon tamoxifen induction. (M) Kaplan–Meier survival curve of homozygous cKO mice after MI (red), homozygous cKO mice after sham surgery (blue), and heterozygous cKO mice after MI (green) over the course of 30 d. (N) Immunofluorescence stainings of FSTL1 (green), Ki67 (red), and TnI (white) in damaged control and homozygous cKO hearts in remote, border, and infarct zone. Nuclei were counterstained with DAPI (blue). (Scale bars: 100 μm.)