Abstract
Background
In the southeastern Senegal, the report of Plasmodium vivax infections among febrile patients in Kedougou constitutes a new emerging health problem.
Methods
Samples from 48 asymptomatic schoolchildren sampled twice a year over 2 years were used to explore the reservoir of P. vivax parasite infections in this region. Both Duffy genotyping and Plasmodium species diagnostic assays were performed.
Results
PCR assays detected Plasmodium genomic DNA in 38.5% (74/192) of samples. Pure P. falciparum and P. vivax infections were identified in 79.7% (59/74) and 20.3% (15/74) of samples, respectively. All schoolchildren were classified as Duffy-negative by genotyping. P. vivax infections were detected in five children: in two children during both years, in one child in 2010 and on May 2011, and only in 2010 for the remaining two children.
Conclusions
This unexpectedly high proportion of P. vivax infections in asymptomatic Duffy-negative children highlights to consider vivax malaria as an emerging problem in Senegal.
Keywords: Malaria, Plasmodium vivax, Asymptomatic carriage, Duffy-negative, Children
Background
Malaria remains a main global cause of death from parasitic diseases threatening approximately half of the world’s population and causing debilitating illness in more than half a million people [1]. Although Plasmodium falciparum remains the deadliest human-infecting Plasmodium species in Africa [1], P. vivax is geographically the most widely distributed malaria parasite, and is yearly responsible of 80–300 million clinical cases, and up to 2.5 billion people are globally at risk [2]. P. vivax has been for long believed to be almost completely absent in large parts of sub-Saharan Africa [3] due to the high prevalence of the Duffy-negative phenotype which is supposed to confer a complete protection against P. vivax malaria [4].
However, this widely accepted dogma has been recently challenged by increasing reports of P. vivax infections in Duffy-negative individuals [5–9], especially in West and Central Africa where previously P. vivax infections were not detected by the conventional microscopy and rapid diagnostic tests [6, 7]. The increasing use of sensitive molecular diagnostics has as expected confirmed high incidences of P. falciparum infections in Africa, but also revealed instances of P. vivax infections in many African countries [5, 7, 10–12].
Vivax malaria is considered to be a chronic infection with a typically mild clinical course [13], due to unique biological features of the parasite including dormant liver-stages relapsing weeks, months, or years after the initial infection, development in mosquito vectors at lower ambient temperatures, and low parasite density. Accordingly, P. vivax infections contribute significantly to the malaria parasite reservoir and potentially serve as a significant source of transmission that could impair malaria control and elimination efforts.
Following our recent reports of P. vivax infections among febrile patients [11] and asymptomatic schoolchildren in Kedougou region, southeastern Senegal [14], this study was undertaken to survey molecular signatures of true P. vivax infections in the same cohort of asymptomatic schoolchildren.
Methods
The samples used in this study were collected from Kedougou region, southeastern Senegal as part of a project investigating arboviruses infections. Details of Kedougou region including malaria indicators, population, climate, rainfall, landscape, and fauna have been previously reported [11, 15]. We tested in total 192 samples collected from 48 asymptomatic schoolchildren (C1 to C48) who were followed during two consecutive years (2010 and 2011), and sampled twice each year (May and November) corresponding to the dry and rainy seasons, respectively. For this study, sera samples were withdrawn from a collection of archived biological specimens established as part of a project investigating asymptomatic and symptomatic arboviruses infections.
The study was examined and approved by the Senegalese National Health Research Committee under the reference 0081MSP/DS/CNRS. A healthcare worker was trained to conduct interviews to explain the study objectives, benefits, and risks to parents/guardians and school administrators before inclusion. Written informed consents were obtained from parents/guardians of children participants.
For this study, samples were tested for Plasmodium spp. infections with two well-validated approaches using genomic DNA (gDNA) extracted from frozen serum samples as previously described [16, 17]. First, the presence of Plasmodium genus and species DNA was tested by nested PCR targeting the 18S ssrRNA genes as previously described [18]. The Plasmodium genus-specific and species-specific PCR amplifications were performed as detailed previously [11]. Second, blinded DNA samples of the entire cohort were sent to an independent laboratory in Cambodia for confirmation of the results using a real-time screening and four species identification PCR assays targeting the Plasmodium cytochrome b gene as described previously by Canier et al. [19].
Sequences to detect SNP in the GATA-1 transcription factor binding site at nucleotide position − 33 (t, wild-type; c, erythrocyte silent) were obtained to determine the Duffy genotype of all individuals, as previously described [5]. PCR products were sent to Macrogen (Seoul, South Korea) for Sanger sequencing. The electropherograms were analyzed on both strands with CEQ2000 Genetic Analysis System software (Beckman Coulter, Brea, CA, USA). Nucleotide sequences were compared to the glycoprotein Duffy group antigen sequence (GenBank accession No. S76830).
Results
At the time of the first sampling (May 2010), children were aged 8 to 11 years old with a mean age of 9. The sex ratio M/F was 1.4 (28/20). All children were asymptomatic (axillary temperature < 37.5 °C) over the four sampling periods.
Of the 192 samples tested (4 × 48), 38.5% (74/192) were found positive by using the genus-specific 18S ssRNA PCR assay (Table 1). The proportions of Plasmodium-positive samples were significantly higher in November (58.3% in 2010 and 43.7% in 2011) compared to May (31.2% in 2010 and 20.8% in 2011, p = 0.007 and p = 0.01, respectively, Fisher’s exact test) for both years. As expected, species-specific nested PCR assay revealed P. falciparum accounted for the majority of infections and was present as pure infection in 79.7% (59/74) of Plasmodium-positive samples. No single or mixed P. malariae or P. ovale infections were detected among the screened samples. We identified additionally 15 pure P. vivax-positive samples from 5 children. The proportions of P. falciparum and P. vivax infections were respectively 20.8% (10/48) and 10.4% (5/48) in May 2010; 47.9% (23/48) and 10.4% (5/48) in November 2010; 14.6% (7/48) and 6.2% (3/48) in May 2011 and 39.6% (19/48) and 4.2% (2/48) in November 2011 (Table 1). All PCR data were confirmed later by Plasmodium cytochrome b gene-based real-time PCR.
Table 1.
Proportion of asymptomatic Plasmodium infections detected among 48 schoolchildren over 2 years sampling period in Kedougou, Senegal
| Sampling period | Plasmodium genus screening | Total | Malaria species screening* | Total | ||
|---|---|---|---|---|---|---|
| Positive n (%) |
Negative n (%) |
P. falciparum
n (%) |
P. vivax
n (%) |
|||
| May 2010 | 15 (31.2) | 33 (68.75) | 48 | 10 (66.7) | 5 (33.3) | 15 |
| November 2010 | 28 (58.3) | 20 (41.66) | 48 | 23 (82.1) | 5 (17.9) | 28 |
| May 2011 | 10 (20.8) | 38 (79.17) | 48 | 7 (70.0) | 3 (30.0) | 10 |
| November 2011 | 21 (43.7) | 27 (56.25) | 48 | 19 (90.5) | 2 (9.5) | 21 |
| Total | 74 (38.5) | 118 (61.5) | 192 | 59 (79.7) | 15 (20.3) | 74 |
*Number and percentage were expressed relative to Plasmodium-positive samples
The proportions of positive P. vivax samples did not vary significantly during follow-ups and were 12.5%, 13.2%, 9.1%, and 10% respectively on May 2010, November 2010, May 2011, and November 2011 (p = 0.28, chi-squared test, Fig. 1). By contrast, proportions of positive P. falciparum increased slightly from May to November: from 20.8% to 47.9% in 2010 (p = 0.009, Fischer’s exact test) and from 14.5% to 39.5% in 2011 (p = 0.01, Fisher’s exact test, Fig. 1).
Fig. 1.
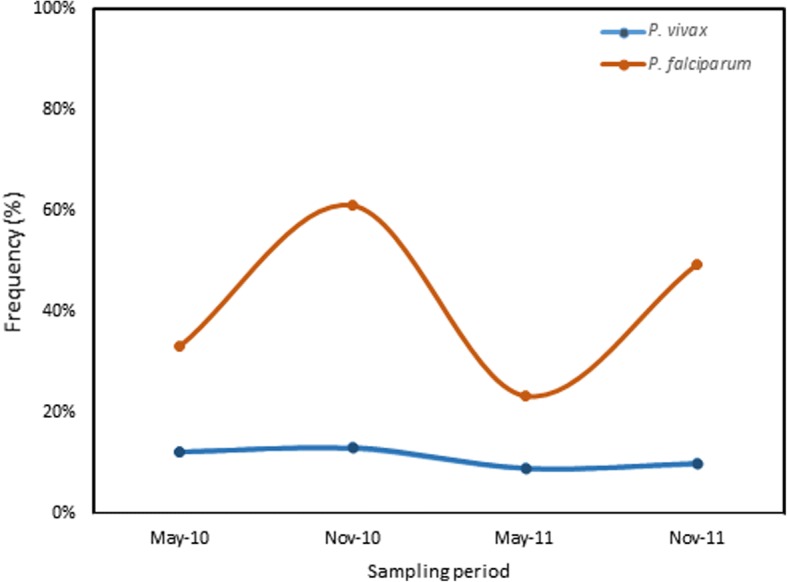
Distribution of positive P. falciparum and P. vivax infections in the cohort during follow-up
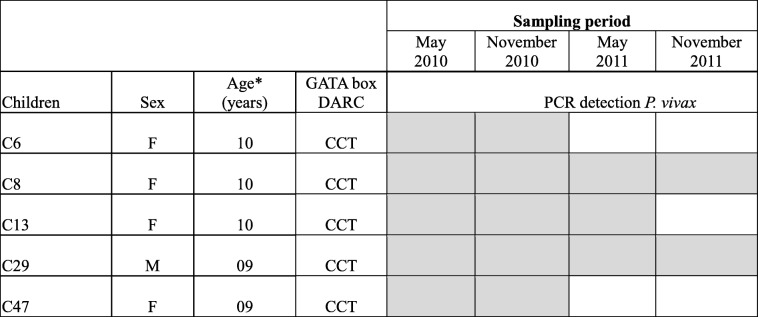
Details regarding the age and sex of the five P. vivax-infected children (identified as C6, C8, C13, C29, and C47) and the dynamics of individual P. vivax carriage across the sampling periods are provided in Table 2. P. vivax parasites were present in all children in May and November 2010, in three children (C8, C13, and C29) in May 2011 and in two children (C8 and C29) in November 2011 (Table 2). All schoolchildren were classified as Duffy negative (FY*BES/*BES) including the five P. vivax-infected ones.
Table 2.
Dynamic carriage of Plasmodium vivax parasites among the five Duffy-negative children across sampling periods (2010–2011), Kedougou, Senegal
*Age at inclusion; gray and white boxes represent the presence and absence of P. vivax, respectively
Discussion
A new vision of P. vivax transmission in Africa has recently emerged with increasing evidence of the parasite’s presence in Duffy-negative individuals [2]. Previous reports, including ours, strongly challenge the paradigm that Duffy-negativity confers a full protection against P. vivax infection and suggest that P. vivax infections in symptomatic subjects probably represent only the tip of the iceberg [7, 14, 20, 21]. Accordingly, survey of P. vivax infections in asymptomatic individuals as in the present study, would provide better estimates of the silent P. vivax parasites reservoir in a given community or setting.
We herein confirm an unexpectedly high proportion (20.3%) of P. vivax infections among asymptomatic schoolchildren in Kedougou region by using molecular detection of P. vivax DNA although this approach cannot firmly confirm the presence of active erythrocytic infections. The possibility of our PCR technique picking up DNA from pre-erythrocytic infections of P. vivax as shown by Abkallo et al. (2015). However, these findings are in line with our previous report detecting 53% positive IgG antibody responses to P. vivax MSP1 antigen with the same samples [15]. In our cohort, P. vivax infections in children C8 and C29 over a period of 2 years might be due to persistent P. vivax parasites. This hypothesis could be supported by the similar proportions of P. vivax infections across sampling periods. Nevertheless, the possibility of relapses or re-infection during follow-up cannot be ruled out. The failure to detect P. vivax parasites in May and November 2011 in children C6 and C47 and in November 2011 in child C13 might be due to the clearance of the parasites from the peripheral blood or a strong reduction of the parasite load at density below the detection limit of our diagnostic methods. In addition, the failure to detect both P. malariae and P. ovale in this study was somehow surprising since recent studies have reported the presence of both species in the area [11, 22].
Finally, the current findings of an important asymptomatic P. vivax reservoir along with the recent report of P. vivax infections among febrile patients in Kedougou region [11] suggest that P. vivax parasites circulate in this area where Duffy negativity is fixed. The growing evidence of P. vivax infections in Kedougou region where transmission of the major malaria parasite species P. falciparum is still active has thus brought in a new paradigm for the control program that might require immediate attention, in order to sustain the limited success gained to date concerning malaria control in the area. Further research is needed to better document the reservoir of P. vivax infections in this population and its relevance to malaria transmission and clinical malaria incidence.
Conclusions
The study reveals an unexpectedly high proportion of P. vivax infections among asymptomatic Duffy-negative children in the Kedougou region. This points out critical needs of further studies investigating factors that drive P. vivax transmission in this particular area where control efforts towards P. falciparum have shown limited impact. We do not know if vivax malaria cases were missed previously or if we are facing to the emergence of strains using alternative Duffy-independent invasion pathways that may lead to an expansion of P. vivax malaria into Duffy-negative populations. Whatever the reason, this is a public health concern which demands attention.
Acknowledgements
The authors thank the population, healthcare workers, parents/guardians and school administrator of participant children and medical authorities in Kedougou region for their support and cooperation in conducting this study.
Funding
This work was supported by the National Institutes of Health [grant Number R01AI069145] and Pasteur Institute of Dakar.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- DARC
Duffy antigen/chemokine receptor
- DNA
Deoxyribonucleic acid
- gDNA
Genomic DNA
- P. falciparum
Plasmodium falciparum
- P. vivax
Plasmodium vivax
- PCR
Polymerase chain reaction
- ssRNA
Small subunit ribonucleic acid
Authors’ contributions
MN, AS, MD, AAS, DM, and ATB conceived and designed the study. MN, RS, SC, and EL performed the experiments. AS, BDS, OF, MD, and AAS organized the recruitment of children and samples collection. MN, DM, and ATB analyzed and interpreted the data, and wrote the manuscript. MN, AAS, DM, and ATB critically revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable
Ethics approval and consent to participate
The collection of human blood samples was performed as described in a previous study [15], and this material has remained stored since then. In the previous study approved under the reference 0081MSP/DS/CNRS, blood samples were collected only after obtaining signed informed consent.
Consent for publication
Biological samples from humans were the reminiscent from a previous project whose data have been already published [15]. Consent forms were obtained from the individuals on that occasion.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World malaria report 2017. Geneva: World Health Organization; 2018. pp. 1–186. [Google Scholar]
- 2.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, Hay SI. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95(6 Suppl):15–34. doi: 10.4269/ajtmh.16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22(8):353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295(6):302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 5.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A. 2010;107(13):5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosario VE, Benito A, Berzosa P, Arez AP. Duffy negative antigen is no longer a barrier to Plasmodium vivax--molecular evidences from the African west coast (Angola and Equatorial Guinea) PLoS Negl Trop Dis. 2011;5(6):e1192. doi: 10.1371/journal.pntd.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niangaly A, Karthigayan G, Amed O, Coulibaly D, Sa JM, Adams M, Travassos MA, Ferrero J, Laurens MB, Kone AK, et al. Plasmodium vivax infections over 3 years in Duffy blood group negative Malians in Bandiagara, Mali. Am J Trop Med Hyg. 2017;97(3):744–752. doi: 10.4269/ajtmh.17-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW, et al. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg. 2006;75(4):575–581. doi: 10.4269/ajtmh.2006.75.575. [DOI] [PubMed] [Google Scholar]
- 9.Wurtz N, Mint Lekweiry K, Bogreau H, Pradines B, Rogier C, Ould Mohamed Salem Boukhary A, Hafid JE, Ould Ahmedou Salem MS, Trape JF, Basco LK, et al. Vivax malaria in Mauritania includes infection of a Duffy-negative individual. Malar J. 2011;10:336. doi: 10.1186/1475-2875-10-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PLoS One. 2015;10(3):e0121763. doi: 10.1371/journal.pone.0121763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niang M, Thiam LG, Sow A, Loucoubar C, Bob NS, Diop F, Diouf B, Niass O, Mansourou A, Varela ML, et al. A molecular survey of acute febrile illnesses reveals Plasmodium vivax infections in Kedougou, southeastern Senegal. Malar J. 2015;14:281. doi: 10.1186/s12936-015-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio JM, Benito A, Roche J, Berzosa PJ, Garcia ML, Mico M, Edu M, Alvar J. Semi-nested, multiplex polymerase chain reaction for detection of human malaria parasites and evidence of Plasmodium vivax infection in Equatorial Guinea. Am J Trop Med Hyg. 1999;60(2):183–187. doi: 10.4269/ajtmh.1999.60.183. [DOI] [PubMed] [Google Scholar]
- 13.Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, Draper CC, Killick-Kendrick R, Garnham PC. A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1976;70(5–6):474–481. doi: 10.1016/0035-9203(76)90132-2. [DOI] [PubMed] [Google Scholar]
- 14.Niang M, Diop F, Niang O, Sadio BD, Sow A, Faye O, Diallo M, Sall AA, Perraut R, Toure-Balde A. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kedougou, southeastern Senegal. Malar J. 2017;16(1):497. doi: 10.1186/s12936-017-2146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, Senghor CS, Dia AT, Weaver SC, Diallo M, Malvy D, et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J. 2016;15(1):47. doi: 10.1186/s12936-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharti AR, Patra KP, Chuquiyauri R, Kosek M, Gilman RH, Llanos-Cuentas A, Vinetz JM: Polymerase chain reaction detection of Plasmodium vivax and Plasmodium falciparum DNA from stored serum samples: implications for retrospective diagnosis of malaria. Am J Trop Med Hyg. 77, 2007/09/11 edn; 2007: 444–446. [PubMed]
- 17.Gal S, Fidler C, Turner S, Lo YM, Roberts DJ, Wainscoat JS. Detection of Plasmodium falciparum DNA in plasma. Ann N Y Acad Sci. 2001;945:234–238. doi: 10.1111/j.1749-6632.2001.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 18.Snounou G. Genotyping of Plasmodium spp. Nested PCR. Methods Mol Med. 2002;72:103–116. doi: 10.1385/1-59259-271-6:103. [DOI] [PubMed] [Google Scholar]
- 19.Canier L, Khim N, Kim S, Sluydts V, Heng S, Dourng D, Eam R, Chy S, Khean C, Loch K, et al. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J. 2013;12:405. doi: 10.1186/1475-2875-12-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Davies DH, Jain A, Lo E, Lee MC, Randall AZ, Molina DM, et al. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand - molecular and serological evidence. Malar J. 2015;14:95. doi: 10.1186/s12936-015-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culleton R, Ndounga M, Zeyrek FY, Coban C, Casimiro PN, Takeo S, Tsuboi T, Yadava A, Carter R, Tanabe K. Evidence for the transmission of Plasmodium vivax in the Republic of the Congo, West Central Africa. J Infect Dis. 2009;200(9):1465–1469. doi: 10.1086/644510. [DOI] [PubMed] [Google Scholar]
- 22.Lucchi NW, Gaye M, Diallo MA, Goldman IF, Ljolje D, Deme AB, Badiane A, Ndiaye YD, Barnwell JW, Udhayakumar V, et al. Evaluation of the Illumigene Malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep. 2016;6:36808. doi: 10.1038/srep36808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.