Abstract
Dilated cardiomyopathy is the most common form of cardiac muscle disease, accounting for approximately 60% of all cardiomyopathies. We proposed to identify histopathological changes of the myocardium in dilative cardiomyopathy. This study comprised a total of 19 cases, represented by myocardial fragments from deceased patients with diagnosis of dilated cardiomyopathy. Histopathological analysis allowed changes to be observed for both myocytes and myocardial interstitial components. We have found a combination of hypertrophic, atrophic and normal myocardocytes, or associated with the presence of hydropic changes. We rarely identified the aspect of myocytosis, cytoplasmic accumulation of lipofuscin pigment or mucinous material, and variable nuclear pleomorphism. At the interstitial level we noticed changes in fibrosis, lipomatosis and rarely the presence of inflammatory infiltrate. Histopathological characteristics of the myocardium in dilated cardiomyopathy are numerous but nonspecific, similar to those in the terminal stages of other cardiac diseases.
Keywords: Dilated myocardiopathy, histopathology
Introduction
Cardiomyopathies are defined as diseases of the myocardium with associated structural and functional abnormalities, which are classified traditionally according to morphological and functional criteria into four categories: dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy/dysplasia.
Dilated cardiomyopathy is the most common form of heart muscle disease, comprising approximately 60% of all cardiomyopathies [1,2].
It is a progressive, diffuse process involving cardiomyocytes from both ventricles, which eventually become dilated and dysfunctional. The myocardium relaxes, which means it stretches and becomes thinner, and consequently the ventricular chamber widens. It is a progressive, diffuse process involving cardiomyocytes from both ventricles, which eventually become dilated and dysfunctional. The myocardium relaxes, which means it stretches and becomes thinner, and consequently the ventricular chamber widens. The histologic features of dilated cardiomyopathy are nonspecific; therefore, it is a microscopic diagnosis of exclusion [3] and identified in various studies only in myocardium of patients with end-stage disease [4].
The present study aims to identify the spread of histopathological changes of myocardiocytes and myocardial interstitium in patients with dilated cardiomyopathy.
Material and Methods
This study included a total of 19 cases from patients diagnosed with dilated cardiomyopathy diagnosed in the Cardiology Clinic of Emergency County Hospital Craiova.
The studied material was represented by myocardial fragments harvested during necropsy, which were fixed in 10% formalin and then processed by the classic histopathological technique using the BioOptica tissue processor and hematoxylin-eosin (HE), periodic acid-Schiff (PAS)-Alcian Blue (AB) and Masson's trichrome staining.
We have followed a number of histopathological parameters such as myocyte atrophy, vacuolar degeneration, myocytolysis, lipofuscin accumulation, mucin accumulation, interstitial fibrosis, lipomatosis and inflammatory infiltrate.
Each of these parameters was rated as focal when it was ≤10%, zonal 10-25% and extended ≥25%. Interpretation was done using the Nikon microscope Eclipse E600 and software program Lucia 5. The written informed consent was obtained from relatives or caretakers of all patients included in this study.
Results
Histopathological features of myocardium from the 19 patients with dilated cardiomyopathy diagnosis allowed changes to be observed for both myocytes and myocardial interstitial components (Table 1).
Table 1.
Distribution of cases according to histopathological parameters
| Histopathological | Focal | Zonal | Extended | |||||
| parameters | Number | % | Number | % | Number | % | ||
| of cases | of cases | of cases | ||||||
| Myocytes atrophy | 3 | 15.8 | 11 | 57.8 | 5 | 26.4 | ||
| Vacuolar degeneration | 10 | 52.6 | 7 | 36.8 | 2 | 10.5 | ||
| Myocytolysis | 7 | 36.8 | 0 | 0 | 0 | 0 | ||
| Lipofuscin accumulation | 9 | 47.3 | 0 | 0 | 0 | 0 | ||
| Mucin accumulation | 6 | 31.5 | 1 | 5.2 | 0 | |||
| Nuclear pleomorphism | 5 | 26.4 | 3 | 15.8 | 1 | 5.2 | ||
| Interstitial fibrosis | 11 | 57.8% | 6 | 31.5% | 2 | 10.5% | ||
| Lipomatosis | 8 | 42.1% | 5 | 26.3% | 0 | 0 | ||
| Inflammatory infiltrate | 5 | 26.4% | 0 | 0 | 0 | 0 | ||
Myocytes had a normal architectural distribution, but showed changes in their number, size, and shape, as well as changes in the cytoplasm and nucleus.
As for the size of myocytes, there were quite large variations. We have observed a combination of hypertrophic, atrophic and normal myocardiocytes, or associated with the presence of degenerative changes.
We have identified atrophy in all investigated cases, but with varying extent.
The most common atrophy was present with zonal character (11 cases, 57.8%), rarely was extensive or focal aspect. Due to the atrophy of the myocytes they had the appearance of long and thin fibers, with a sinuous path, with a characteristic appearance of "corrugated fibers" (Fig.1), separated from each other by loose spaces, a suggestive aspect for edematous infiltration of the interstitia.
Figure 1.
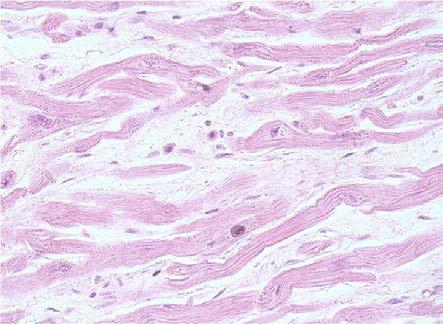
Dilated cardiomyopathy, atrophy with "corrugated" fibers appearance, HE staining, x10
Myocytes cytoplasm has shown some changes, such as vacuolar degeneration, myocytosis, reduction in myofibril counts, lipofuscin or mucin pigment accumulation.
Changes in vacuolar degeneration were present in all investigated cases but with variable spread. In 10 cases (53%) the appearance was only focal, affecting a small number of myocytes. In 7 cases (37%) the changes included larger areas with a zonal character, and in 2 cases it was diffused into the myocardial fibers (10%). Changes in vacuolar degeneration had variable cytoplasmic morphology and topography.
Most often, we observe the presence of several empty vacuoles, with irregular contours and imprecisely delimited, isolated or confluent, dispersed in the cytoplasm (Fig.2).
Figure 2.
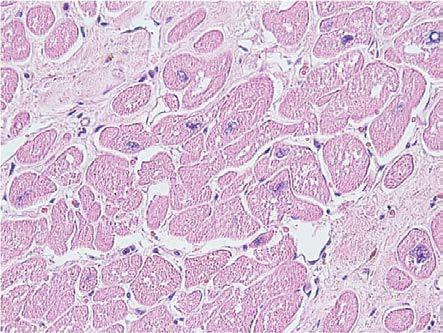
Dilated cardiomyopathy, hydropic degeneration, HE staining, x400
Myocytolysis was rarely identified, recognized by muscle fibers fragmentation. The lesion had only focal character, affecting isolated myocytes or small groups.
In 9 cases we noted the accumulation of the lipofuscin pigment in the form of brown granules of variable size, preferentially perinuclear disposed. The cytoplasmic accumulation of mucinous material, with slightly basophilic tinctoriality, PAS positive, was rarely present (7 cases, 37%) and almost always focal, only in one case the modification being zonal (Fig.3, Fig.4).
Figure 3.
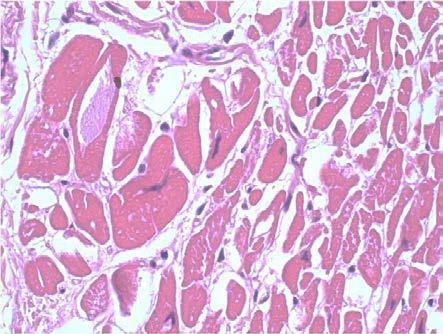
Dilated cardiomyopathy, mucinous degeneration, HE staining, x400
Figure 4.
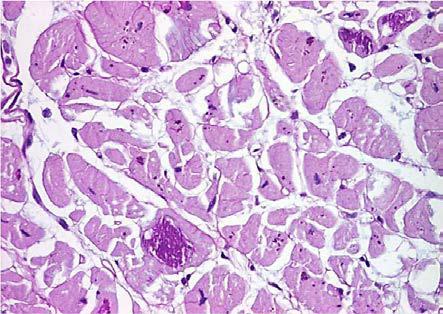
Dilated cardiomyopathy, mucinous degeneration, PAS staining, x400
Myocytes nuclei also had changes, both in form and in tinctoriality. Their dimensions were variable, sometimes large and hyperchromatic, with bizarre forms and variable pleomorphism from mild (5 cases) to moderate (3 cases) and to severe (1 case) (Fig.5).
Figure 5.
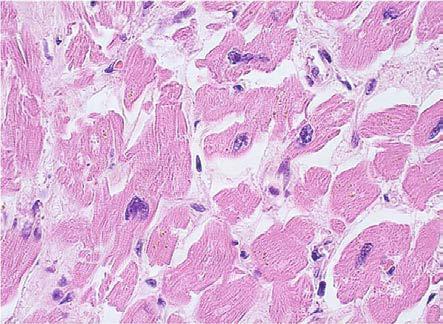
Dilated cardiomyopathy, nuclear pleiomorphism and perinuclear lipofuscin, HE staining, x400
At the interstitial level we noticed changes in fibrosis, lipomatosis and rarely the presence of inflammatory infiltrate.
Fibrosis was diffuse or focal, with interstitial or sometimes perivascular topography. In interstitial fibrosis, we noticed the presence of the fibrillar collagen in intermuscular spaces that are normally devoid of collagen. The distribution pattern was quite variable, from a fine peri-myocyte distribution to massive scars, sometimes similar to those in ischemic cardiopathy. Collagen has individually surrounded the myocardial fibers that have a "ragged" appearance due to the corrugated cell membrane. In the case of perivascular fibrosis, collagen has accumulated in the adventitia of intramedullary coronary arteries and arterioles, otherwise untouched. At the interstitial level we noticed changes in fibrosis, lipomatosis and rarely the presence of inflammatory infiltrate.
Depending on the magnitude of the change, the analyzed cases presented minimal fibrosis with a focal character in 11 cases, moderate that had a zonal character in 6 cases, or extended and diffuse in 2 cases. The aspect was subsequently confirmed by Masson's trichrome staining (Fig.6, Fig.7).
Figure 6.
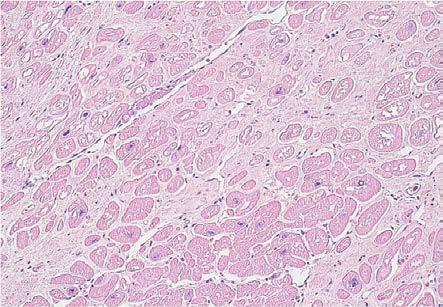
Dilated cardiomyopathy, moderate fibrosis and diffuse hydropic degeneration, HE staining, x100
Figure 7.
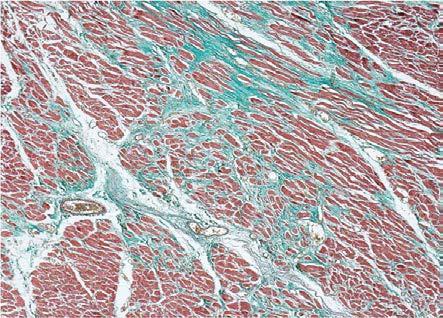
Dilated cardiomyopathy, moderate fibrosis, Masson's trichrome staining, x40
Sometimes, we noticed the presence of fatty tissue infiltrate (lipomatosis) with focal character. The appearance was identified in 13 cases, where adipocytes, generally of small size, were disposed isolated or in small groups between the myocardial fibers.
Inflammatory infiltrate composed of rare lymphocytes was rarely identified, with a dispersed appearance in fibro-collagen tissue. We also identified rare mast cells, confirmed by the PAS-AB staining (Fig.8).
Figure 8.
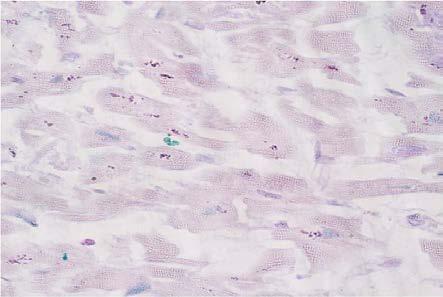
Dilated cardiomyopathy, rare mastocytes, Alcian Blue PAS staining, x400
Discussions
The main components of myocardial structure are cardiomyocytes and the extracellular matrix (EM). Cardiomyocytes account for approximately 76% of myocardial volume and the extracellular matrix is approximately 24% [5]. In dilated cardiomyopathy, both components of both myocardial and extracellular matrix were identified. The aspects described on endomyocardial biopsies from patients with dilated cardiomyopathy range from minimal differences in the size of myocytes to those typical of the disease with marked variations in the size of myofibrils, loss of myofibrils and interstitial fibrosis [3,6]. In one study, the authors reported the association of interstitial fibrosis with hypertrophy of myofibrils, along with degeneration and atrophy of the myocytes in 60% of the investigated dilated cardiomyopathy [7].
The content of fibrous tissue in the myocardium of patients with dilated cardiomyopathy is associated with a combination of hypertrophic, atrophic and normal myocardiocytes or with the presence of degenerative changes, which led to the hypothesis of a vicious circle [8].
According to it, modified myocytes can release substances that stimulate the extracellular matrix growth but in turn its extension may overload myocytes and the continuous increase of the collagen network from dilated cardiomyopathy being explained by the progressive degeneration of cardiomyocytes [9].
Myocytic atrophy is frequently mentioned between the histopathological features of dilated cardiomyopathy [6], being defined by the presence of elongated, thin myocytes with a narrow cytoplasmic border, their corrugated appearance constituting an indicative image of their overextension. Corrugated myocytes, especially when associated with focal edema, are characteristic changes in acute myocardial ischemia [10], but have also been described in patient with dilated cardiomyopathy [11,12]. In our study, we observed quite large variations in the myocardial size, along with the atrophic myocyte areas characterized by the reduction of the cross-sectional diameter, as well as the areas with normal or sometimes even hypertrophic myocytes. In one study, the percentage of myocytes decreased from 78.10±7.34 in control cases to 63.39+/-9.22 in dilated cardiomyopathy (p<0.05) and 47.28+/-15.01 in arrhythmogenic right ventricular cardiomyopathy (p<0.01) [13].
We identified atrophy of myocardial cells in all investigated cases, but with varying distribution, respectively focal length in 15.8% of cases, with zonal character in 57.8% of cases and diffuse appearance in 26.4% of these. Yonesaka et al. reported myocardial atrophy in only 2 out of 16 patients with idiopathic dilated cardiomyopathy studied [14]. Another study reported in dialyzed patients with dilated cardiomyopathy: severe hypertrophy of myocytes with a mean myocytic diameter of 37.6±10.5 vs. 25.6±7.7 in control cases (p=0.001) and disruption of myocytes in 30% of cases of dilated cardiomyopathy [15].
Normal myocardiocytes have a length of 100 μm and a diameter of 10-25μm [16,17]. Myocardial lesions associated with corrugated atrophy of myocytes consist of the presence of myocardial cells with a diameter of less than 10μm, with a corrugated appearance, which comprises at least half the thickness of the myocardial specimens in the upper and lower portions of the left ventricle (LV). In one study, the authors reported an average diameter of 22.89μm (3.30) for myocardocytosis in dilated cardiomyopathy patients compared to 16.74μm (1.17) in control cases [18].
The vacuolar degeneration of myocytes is frequently mentioned, with the appearance of cytoplasmic vacuoles or sometimes as a perinuclear halo [19].
In the study, the presence of vacuolar degeneration of myocytes was observed in all cases, often focal. Myocitolysis is not a characteristic change in dilated cardiomyopathy [3].
Johnson et al. did not find any case of dilated cardiomyopathy with myocytosis in cases of endomyocardial biopsy, but the appearance was identified and reported in 9% of necropsy cases [20].
In the study we observed the presence of myocytolysis in only 7 cases and it was only focal.
Nuclear pleomorphism is frequently described, with myocytes having dysmetric and dysmorphic nuclei [6,19].
For the analyzed cases we noticed the nuclear pleomorphism in 9 of the analyzed cases, frequently with focal character. Arbustini et al. analyzed the large nuclei and bizarre morphology, and found that the width of the myocytes, nuclear diameter, and nucleo -sarcoplasmic ratio were significantly higher in the group compared to cases lacking these modifications [21].
The mean values were 36±5μm, 14±3μm and 0.41±0.08 respectively for the cases with nuclear changes, compared to 20±8μm, 7±2μm and 0.37±0.08 for cases without nuclear modification [21].
In one study, the authors reported statistically significant differences in the number of nuclei/mm2 of myocardial tissue, diameter and area [18].
In another study it was suggested that nuclear abnormalities may constitute the main event in the pathogenesis of dilated cardiomyopathy, with authors reporting lowered nuclear density (18%, p<0.05), but the area of the nuclear profile significantly increased (85%, p<0.001), the nucleus/cytoplasmic relationship being thus modified [22].
Interstitial changes have been described in several studies in patients with dilated cardiomyopathy. These consist of fibrosis, focal fat infiltration, and sometimes the presence of focal inflammatory infiltration.
The most typical change in dilated cardiomyopathy is the development of interstitial and perivascular fibrosis of varying degrees [6,23].
Fibrosis in dilated cardiomyopathy, as in other cardiac diseases, is an essential component of the myocardial remodeling process but also has some distinctive features. Obviously, the composition and properties of the extracellular matrix affects the mechanical properties of the myocardium during systole and diastole, and alterations that lead to myocardial fibrosis, even in intact cardiomyocytes, may lead to heart failure [24], perhaps due to limited supply of oxygen to cardiomyocytes and distortion of electrical contacts between them.
Quantification of collagen showed up to 4-fold increase from normal collagen volume, with a decrease in mature reticulated collagen, correlated with increased neutrophil collagenase activity [25].
After other studies in advanced stages of the disease, the collagen concentration is increased by two [26] to five times [27].
Another study reported a percentage of 18.71% (7.76) fibrosis in patients with dilated cardiomyopathy compared to 13.39% (9.55) in normal patients [18].
The percentage of collagen volume in the LV myocardium is significantly higher in patients with dilated cardiomyopathy compared to those with ischemic cardiomyopathy [1].
The increase in the collagen fraction is correlated with the degree of LV dilatation [28].
One study reported a correlation between the percentage of fibrosis, the LV ejection fraction and the functional heart rate [29].
In cases where fibrosis was <10%, there were high ejection fraction values and are included in class I of global functional capacity, while cases with fibrosis >10% are in Class III and IV of global functionality capacity and have low ejection fraction [29].
Johnson et al. reported that fibrosis in patients with dilated cardiomyopathy was in only 28% of endomyocardial biopsy specimens and in 50% of necropsy sections [20].
In contrast, Sekiguchi et al. found the presence of fibrosis in 100% of cases in both endomyocardial biopsy and necropsy specimens [30].
The volume of interstitial tissue density was nearly twice as high in patients with dilated cardiomyopathy who died 3 years after surgery (reductive annuloplasty) compared to patients who survived 7 years after this time (0.20±0.023 times 0.13±0.02, p<0.01) [31].
Another study reported extensive fibrosis in dialysis patients with dilated cardiomyopathy, with a mean area of LV fibrosis of 22.3±18.4% vs. 21.3±14.6% [15].
The rate of non-fatal survival at 3 years for dialysis patients with severe fibrosis (over 30%) was 42%, while for patients without severe fibrosis it was 82% (p=0.03), the authors concluding that the degree of LV fibrosis is a strong predictor of cardiac death [15].
A comparative study that looked at the extent of fibrosis in various cardiac conditions reported the percentage of LV fibrosis in hearts from patients with dilated cardiomyopathy (12.9±8.6%) is significantly lower than in patients with hypertrophic cardiomyopathy that mimicked dilated cardiomyopathy (35.8±11.9%, p<0.01) and those with myocardial infarction (38.4±8.0%, p<0.01) [32].
In dilated cardiomyopathy, fibrosis formation is a continuous process, unlike myocardial ischemia and hypertrophic cardiomyopathy where its formation stops after reaching a certain degree [33,34,35].
Focal accumulation of adipose tissue is a common phenomenon in dilated cardiomyopathy [4,36,37], and has been associated with other features of the disease, such as fibrosis volume and LV function [36].
In our study we have observed the presence of lipomatosis 13, of which 8 cases with focal character and 3 cases with zonal aspect.
In a study, fatty tissue ranged from 0.33±1.44 in control cases to 0.07±0.31 in dilated cardiomyopathy and 13.30±17.30 in right ventricular cardiomyopathy (p<0.05) [13].
The authors consider the presence of fatty tissue as a feature of severe ventricular cardiomyopathy, present in 67% of these patients, compared with only 6% of the control cases and patients with dilated cardiomyopathy [13].
Inflammatory cell infiltration, including lymphocytes [6] and mast cells [38,39], tend to be numerically elevated in fibrosis areas, but distinct inflammatory infiltrations as well as necrosis of myocytes are usually absent [3].
One study noted the presence of inflammatory infiltration in 2 of the 15 investigated cases [7].
In our study, mastocytes have only rarely been identified with focal character. In another study on the density of mast cell infiltration, the authors reported the highest density numerical area of mast cells found in the active myocarditis (3.92/mm2, Standard deviation-SD=1.84), followed by borderline myocarditis (2.76/Mm2, SD=1.66), dilated cardiomyopathy (1.56/mm2, SD=0.45) and control group (0.77/mm2, SD=0.19) [38].
In addition, the authors believe that mast cells might be involved in modulation of the fibrosis response due to the fact that they tended to be associated with fibrosis areas [38].
Conclusion
Histopathological aspects of the myocardium in dilated cardiomyopathy are numerous but unspecific and variable in amplitude, similar to those encountered in the final stages of other cardiac diseases.
References
- 1.Sisakian H. Cardiomyopathies: Evolution of pathogenesis concepts and potential for new therapies. World J Cardiol. 2014;6(6):478–494. doi: 10.4330/wjc.v6.i6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nighute SS, Shiddapur GS, Mahashaabde M, Ranpara CK, Shinde SB. Study of grading of severity of heart failure in patients with dilated cardiomyopathy. Indian J Basic Appl Med Res. 2017;6(2):190–194. [Google Scholar]
- 3.McNally EM, Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res. 2017;121(7):731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Leeuw, Ruiter DJ, Balk AH, de Jonge, Melchers WJ, Galama JM. Histopathologic findings in explantedheart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14(5):299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 5.Frank JS, Langer GA. The myocardial interstitium: its structure and its role in ionic exchange. J Cell Biol. 1974;60:586–601. doi: 10.1083/jcb.60.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershberger RE, Morales A. In: MDilated Cardiomyopathy Overview, 2007. Pagon RA, Adam MP, Ardinger HH, et al., editors. Seattle (WA): GeneReviews [Internet]; 2018. [Google Scholar]
- 7.Almazán A, Murillo H, Badui E. Usefulness of endomyocardial biopsy in myocarditis and dilated cardiomyopathy. Arch Inst Cardiol Mex. 1989;59(6):573–577. [PubMed] [Google Scholar]
- 8.Schaper J, Speiser B. The extracellular matrix in the failing human heart. Basic Res Cardiol. 1992;87(Suppl 1):303–309. doi: 10.1007/978-3-642-72474-9_26. [DOI] [PubMed] [Google Scholar]
- 9.Kapelko VI. Extracellular matrix alterations in cardiomyopathy: The possible crucial role in the dilative form. Exp Clin Cardiol. 2001;6(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Eichbaum F. Wavy' myocardial fibers in spontaneous and experimental adrenergic cardiopathies. Cardiology. 1975;60(6):358–365. doi: 10.1159/000169735. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy PM, Nakatani S, Vargo R, Kottke-Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann Thorac Surg. 1995;59(3):609–613. doi: 10.1016/0003-4975(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani S1, McCarthy PM, Kottke-Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Left ventricular echocardiographic and histologic changes: impact of chronic unloading by an implantable ventricular assist device. J Am Coll Cardiol. 1996;27(4):894–901. doi: 10.1016/0735-1097(95)00555-2. [DOI] [PubMed] [Google Scholar]
- 13.Angelini A, Thiene G, Boffa GM, Calliari I, Daliento L, Valente M, Chioin R, Nava A, Volta SD, Calliaris I. Endomyocardial biopsy in right ventricular cardiomyopathy. Int J Cardiol. 1993;40(3):273–282. doi: 10.1016/0167-5273(93)90011-5. [DOI] [PubMed] [Google Scholar]
- 14.Yonesaka S, Becker AE. Dilated cardiomyopathy: diagnostic accuracy of endomyocardial biopsy. Br Heart J. 1987;58(2):156–161. doi: 10.1136/hrt.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005;67(1):333–340. doi: 10.1111/j.1523-1755.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 16.Göktepe S, Abilez OJ, Parker KK, Kuhl E. A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J Theor Biol. 2010;265(3):433–442. doi: 10.1016/j.jtbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Mol Cell Cardiol. 1996;28(7):1463–1477. doi: 10.1006/jmcc.1996.0137. [DOI] [PubMed] [Google Scholar]
- 18.Di Somma, Marotta M, Salvatore G, Cudemo G, Cuda G, De Vivo, Di Benedetto, Ciaramella F, Caputo G, de Divitiis. Changes in myocardial cytoskeletal intermediate filaments and myocyte contractile dysfunction in dilated cardiomyopathy: an in vivo study in humans. Heart. 2000;84(6):659–667. doi: 10.1136/heart.84.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basso C, Ronco F, Marcus F, Abudureheman A, Rizzo S, Frigo AC, Bauce B, Maddalena F, Nava A, Corrado D, Grigoletto F, Thiene G. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J. 2008;29(22):2760–2771. doi: 10.1093/eurheartj/ehn415. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RA, Palacios I. Dilated cardiomyopathies of the adult (second of two parts) N Engl J Med. 1982;307(18):1119–1126. doi: 10.1056/NEJM198210283071804. [DOI] [PubMed] [Google Scholar]
- 21.Arbustini E, Gavazzi A, Pozzi R, Grasso M, Pucci A, Campana C, Graziano G, Martinetti M, Cuccia M, Salvaneschi L, et al. The morphologic spectrum of dilated cardiomyopathy and its relation to immune-response genes. Am J Cardiol. 1989;64(16):991–995. doi: 10.1016/0002-9149(89)90796-0. [DOI] [PubMed] [Google Scholar]
- 22.Scholz D, Diener W, Schaper J. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5(2):127–138. [PubMed] [Google Scholar]
- 23.Sanderson JE, Olsen EG, Gatei D. Dilated cardiomyopathy and myocarditis in Kenya: an endomyocardial biopsy study. Int J Cardiol. 1993;41(2):157–116. doi: 10.1016/0167-5273(93)90156-b. [DOI] [PubMed] [Google Scholar]
- 24.Maisch B. Fat deposition in dilated cardiomyopathy assessed by CMR. Ventricular remodeling. Cardiology. 1996;87(Suppl 1):2–10. doi: 10.1159/000177160. [DOI] [PubMed] [Google Scholar]
- 25.Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol. 1996;148(5):1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop JE, Greenbaum R, Gibson DG, Yacoub M, Laurent GJ. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990;22(10):1157–1165. doi: 10.1016/0022-2828(90)90079-h. [DOI] [PubMed] [Google Scholar]
- 27.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57(10):816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 28.Sisakian S, Gukasian LV, Mkrtshian LG, Kamalov GG, Avakian SA. The role of quantitative determination of the volume fraction of interstitial collagen and fibronectin in the pathogenesis of myocardial remodeling in dilated cardiomyopathy. Klin Med (Mosk) 2001;79(6):24–26. [PubMed] [Google Scholar]
- 29.Agapitos E, Kavantzas N, Nanas J, Margari Z, Bakouris M, Kassis K, Panolaridis A, Davaris P. The myocardial fibrosis in patients with dilated cardiomyopathy. The application of image analysis in the myocardial biopsies. Gen Diagn Pathol. 1996;141(5-6):305–311. doi: 10.1117/12.238788. [DOI] [PubMed] [Google Scholar]
- 30.Sekiguchi M, Hiroe M, Morimoto S. On the standardization of histopathological diagnosis and semiquantitative assessment of the endocardium obtained by endomyocardial biopsy. Bull Heart Inst Jpn. 1980;1979-1980:55–85. [Google Scholar]
- 31.Zorc M, Vraspir-Porenta O, Zorc-Pleskovic R, Radovanović N, Petrovic D. Apoptosis of myocytes and proliferation markers as prognostic factors in end-stage dilated cardiomyopathy. Cardiovasc Pathol. 2003;12(1):36–39. doi: 10.1016/s1054-8807(02)00134-5. [DOI] [PubMed] [Google Scholar]
- 32.Ohtani K, Yutani C, Nagata S, Koretsune Y, Hori M, Kamada T. High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1995;25(5):1162–1169. doi: 10.1016/0735-1097(94)00529-y. [DOI] [PubMed] [Google Scholar]
- 33.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Sonnenblick EH, Olivetti G, Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27(1):291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 34.Gabler U1, Berndt A, Kosmehl H, Mandel U, Zardi L, Müller S, Stelzner A, Katenkamp D. Matrix remodelling in dilated cardiomyopathy entails the occurrence of oncofetal fibronectin molecular variants. Heart. 1996;75(4):358–362. doi: 10.1136/hrt.75.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura A, Kusachi S, Nogami K, Yamanishi A, Kajikawa Y, Hirohata S, Tsuji T. Tenascin expression in endomyocardial biopsy specimens in patients with dilated cardiomyopathy: distribution along margin of fibrotic lesions. Heart. 1996;75(3):291–294. doi: 10.1136/hrt.75.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu M, Zhao S, Jiang S, Yin G, Wang C, Zhang Y, Liu Q, Cheng H, Ma N, Zhao T, Chen X, Huang J, Zou Y, Song L, He Z, An J, Renate J, Xue H, Shah S. Fat deposition in dilated cardiomyopathy assessed by CMR. JACC Cardiovasc Imaging. 2013;6(8):889–898. doi: 10.1016/j.jcmg.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Basso C, Perazzolo MM, Thiene G. Myocardial clefts, crypts, or crevices: once again, you see only what you look for. Circ Cardiovasc Imaging. 2014;7(2):217–219. doi: 10.1161/CIRCIMAGING.114.001744. [DOI] [PubMed] [Google Scholar]
- 38.Petrovic D, Zorc M, Zorc-Pleskovic R, Vraspir-Porenta O. Morphometrical and stereological analysis of myocardial mast cells in myocarditis and dilated cardiomyopathy. Folia Biol (Praha) 1999;45(2):63–66. [PubMed] [Google Scholar]
- 39.Batlle M, Pérez-Villa F, Lázaro A, Garcia-Pras E, Ramirez J, Ortiz J, Orús J, Roqué M, Heras M, Roig E. Correlation between mast cell density and myocardial fibrosis in congestive heart failure patients. Transplant Proc. 2007;39(7):2347–2349. doi: 10.1016/j.transproceed.2007.06.047. [DOI] [PubMed] [Google Scholar]


