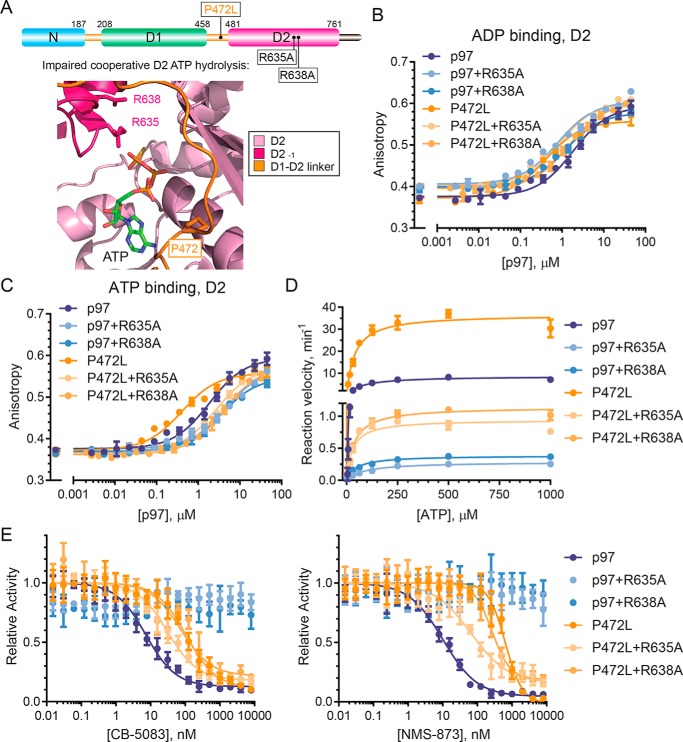
Figure 5.
P472L mutation enhances cooperative D2 ATP binding and hydrolysis. A, p97 schematic (top) and D2 catalytic pocket model (bottom, PDB code FTM) show arginine finger residues Arg-635 and Arg-638 from an adjacent D2 domain involved in cooperative D2 ATP hydrolysis. The affinities of ADP (B) or ATP (C) to the D2 domains of the indicated p97 or P472L mutant proteins were measured by fluorescence polarization (n = 2, S.D. error). D, kinetic properties of p97 and the P472L mutant proteins in the presence or absence of R635A or R638A were obtained from ATP titration experiments (n = 4, S.D. error). E, sensitivities of p97 and the P472L mutant proteins to CB-5083 and NMS-873 were tested in inhibitor titration experiments (n = 4, S.D. error). Dissociation constants for ATP and ADP binding to D2, enzyme kinetic properties, and IC50 values are summarized in Table 3.