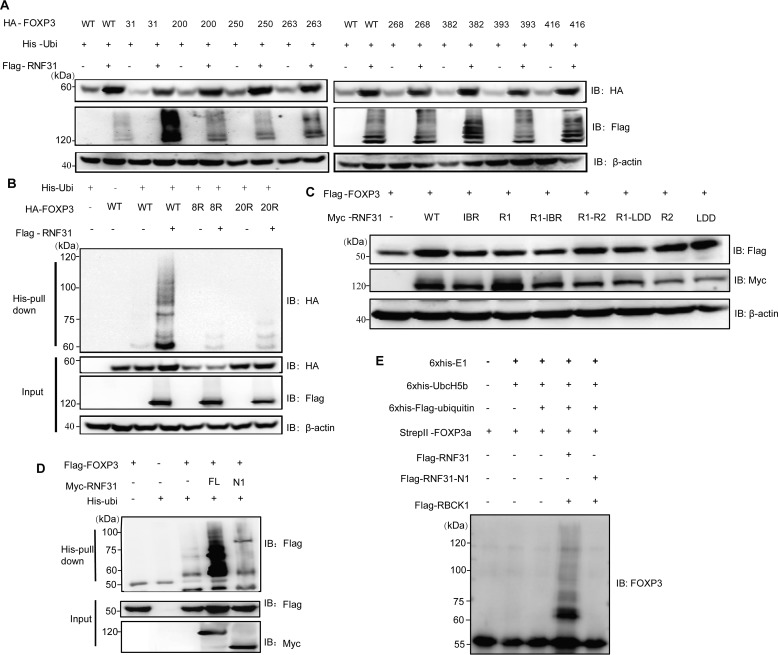
Figure 4.
Characterization of lysine residues of FOXP3 targeted by RNF31. A, immunoblot analysis for the identification of lysine residues of FOXP3 targeted by RNF31 in the HEK-293T cell lysate transfected with FLAG-RNF31, His-ubiquitin, HA–tagged WT FOXP3, and mutant FOXP3 (with retention of one lysine residue). B, immunoblot analysis of ubiquitin chain formation on FOXP3 in HEK-293T cell lysate transfected with FLAG-RNF31, His-ubiquitin, HA–tagged WT FOXP3, and mutant FOXP3–8R/20R (with 8 lysine-to-arginine substitutions as indicated above, or with 20 lysine-to-arginine substitutions). C, immunoblot analysis for identifying RNF31 enzymatically inactive mutants in the HEK-293T cell lysate transfected with FLAG-FOXP3, Myc–tagged WT RNF31, or mutant RNF31 as indicated. D, immunoblot analysis of ubiquitin chain formation on FOXP3 in HEK-293T cell lysates transfected with FLAG-FOXP3, His-Ubiquitin, Myc–tagged WT RNF31 or N1 RNF31 truncation mutant as indicated. E, immunoblot analysis of ubiquitin chain formation on FOXP3 in a cell–free system with His6-E1 (100 ng), His6-E2 (200 ng), His6-FLAG ubiquitin (10 μg), StrepII FOXP3 (500 ng), 2× FLAG RNF31 (500 ng), 2× FLAG-N1-RNF31 (500 ng), and 2× FLAG RBCK1 (500 ng) being mixed in the reaction buffer, which was terminated by adding SDS protein loading buffer.