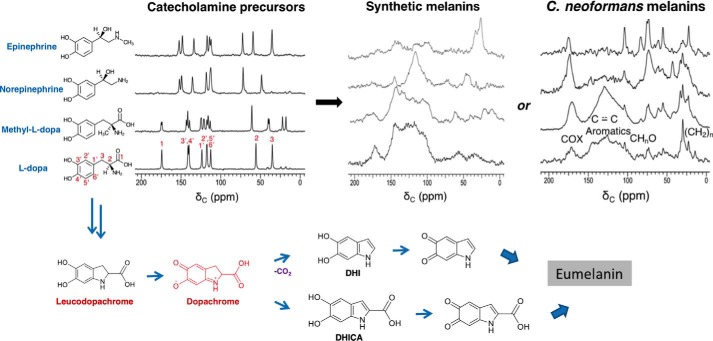
Figure 1.
From upper left to right are shown the 150 MHz solid-state CPMAS 13C NMR spectra of catecholamine precursors, the corresponding melanins obtained by cell-free autopolymerization, and the corresponding melanin ghosts obtained by fungal biosynthesis; below are the proposed structural intermediates in the conversion of l-DOPA to a melanin pigment. C. neoformans melanin spectra and the proposed synthetic scheme are adapted from Ref. 9. This research was originally published in Biochemistry. Chatterjee, S., Prados-Rosales, R., Frases, S., Itin, B., Casadevall, A., and Stark, R. E. Using solid-state NMR to monitor the molecular consequences of Cryptococcus neoformans melanization with different catecholamine precursors. Biochemistry. 2012; 51: 6080–6088. © American Chemical Society. Each chemical constituent contains the 13C isotope at natural abundance (1.1%).