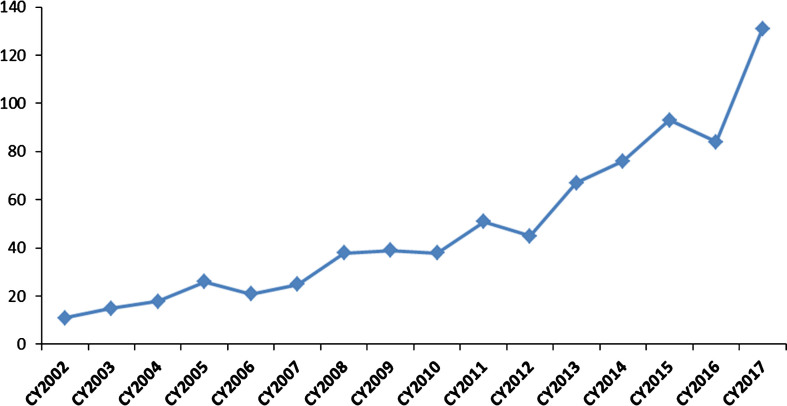
Fig. 2.
Investigational New Drug (IND) applications with development programs that included study populations with rare diseases. The graph depicts the rising numbers of new IND applications with rare disease populations for cell, gene, tissue-based, and other biological products submitted to OTAT (formerly Office of Cellular, Tissues, and Gene Therapies – OCTGT) in calendar years 2002–2017.