Abstract
The goal of this study was to explore the association of beta-amyloid accumulation and cerebrovascular response (CVR) in cognitively normal older adults. Beta-amyloid accumulation was characterized with [18F] Florbetapir positron emission tomography scans. CVR was calculated as middle cerebral artery blood flow velocity change from rest to moderate intensity exercise. We found that individuals with elevated beta-amyloid aggregation had a blunted CVR (n = 25, age 70.1 ± 4.8; 3.3 ± 3.7 cm/s) compared to non-elevated individuals (n = 45, age 72.0 ± 4.9; 7.2 ± 5.0 cm/s, p < 0.001). Further, greater beta-amyloid burden was linearly associated with less CVR across all participants (b = −11.7, p < 0.001). Greater CVR and less beta-amyloid burden were associated with processing speed (p < 0.05). This study is the first to show that CVR from rest to exercise is blunted across increased global beta-amyloid burden.
Keywords: Alzheimer's disease, cerebrovascular, cognitive aging, vascular dementia, ultrasound
Introduction
There is evidence in animal models that the presence of beta-amyloid peptide aggregation, a hallmark pathology of Alzheimer's disease,1 may interfere with endothelial-dependent response of the cerebral arteries and impair cerebrovascular autoregulation.2,3 Transgenic mice expressing the highest levels of beta-amyloid showed the greatest disruption in cerebrovascular autoregulation.2 Insofar as impaired cerebrovascular function is one factor by which beta-amyloid accumulation causes neurodegeneration,4 replication in humans would have important implications regarding brain health.
Several studies have focused on the interaction of cerebral blood flow and beta-amyloid aggregation in Alzheimer's disease.5–8 One study reported no significant differences between resting cerebral artery blood flow between those characterized as mild cognitive impairment and healthy controls.9 However, the authors did report that a task-enhanced challenge or “brain stress test” improved group discrimination in cerebral blood flow. It may be that probing the responsiveness of a mechanism, such as regulation of perfusion during a cognitive or physiological challenge, will reveal deficits that are not apparent in resting conditions. That is, adding a physiologic challenge such as exercise may increase the likelihood to detect subtle vascular changes in preclinical or early disease states.9
Exercise presents a physiologic challenge to the cerebrovascular system due to rapid increases in MAP, increased sympathetic activity, and greater cardiac output to meet metabolic demand.10,11 In this exploratory study, we employed a moderate intensity exercise protocol to assess cerebrovascular response (CVR) in the middle cerebral artery (MCA).12 Using established criteria for indexing cerebral amyloid burden based on PET findings with [18F] Florbetapir burden,13,14 we divided the participants into two groups (elevated, non-elevated) to examine group differences in CVR. We hypothesized that individuals characterized as beta-amyloid elevated would have a lower CVR than non-elevated participants. We also hypothesized that greater beta-amyloid burden would be associated with lower CVR. As a secondary hypothesis, we expected that greater CVR would be associated with better cognitive performance.
Material and methods
Participants
Individuals were recruited as part of an ongoing Alzheimer's prevention program to characterize cerebral beta-amyloid burden in cognitively normal older adults. Inclusion criteria for the program were: (1) 65–90 years of age; (2) classified as cognitively normal/non-demented based on neuropsychological testing and a Clinical Dementia Rating (CDR) = 0; (3) sedentary or underactive lifestyle; (4) completion of [18F] Florbetapir positron emission tomography (PET) scan within six months of our experimental procedures. Exclusion criteria were: (1) Diagnostic and Statistical Manual of Mental Disorders-IV defined drug or alcohol abuse within the prior two years; (2) clinically significant depression or anxiety; (3) insulin-dependent diabetes; (4) myocardial infarction or symptoms of coronary artery disease within the prior two years; (5) acute decompensated congestive heart failure or class IV heart failure; (6) major orthopedic disability; (7) inability to exercise due to pain or restrictions from physician.
Standard protocol approvals, registrations, and patient consents
All individuals provided written informed consent. The KU Institutional Review Board provided approval for all study procedures, which complied with the Declaration of Helsinki.
Cognitive evaluation testing
Participants were evaluated for dementia using the Uniform Data Set (UDS) neuropsychological test battery and the CDR scale employed by the United States Alzheimer's Disease Center network.15,16 Each person completed a standard in-person clinical and cognitive evaluation to exclude dementia or mild cognitive impairment. A trained clinician completed a CDR16 and a psychometrician performed the neuropsychological test battery.17 Clinical and cognitive data were reviewed at a consensus diagnostic conference,18 and cognitively normal participants were defined as having a CDR 0 and no clinically significant deficits on neuropsychological testing.
Tests in the UDS can be aggregated into cognitive domains (memory, executive function, language, processing speed, and attention) and normed to a cognitively normal sample of older adults.19 We took this approach for our analysis of cognitive performance. However, during our project, the National Alzheimer's Coordinating Center changed certain tests in the battery17 and logistically we were unable to maintain a uniform battery. Consequently, we have used the summed free recall of the Free and Cued Selective Reminding Test20 for the Memory domain in all participants. We normed this value to a similar population as reported previously.21
Brain imaging
PET images were obtained on a GE Discovery ST-16 PET/CT scanner. Two 5-min duration PET brain frames were acquired continuously, approximately 50 min after [18F] Florbetapir (370 MBq) administration. Head movement was minimized by use of self-adherent wrap across the forehead. Frames were then summed and attenuation corrected.
We performed two analyses of beta-amyloid burden: (1) a binary categorization of elevated versus non-elevated individuals for group assignment using a well-developed process using three trained raters,14,22,23 (2) a continuous quantitative measure (mean SUVR of six regions of interest). Quantitative analysis was performed using MIMneuro software (v 6.0.5, MIM Software Inc.) as reported previously.13 Briefly, nine-parameter affine registration was used first to align raw reconstructed the image to three [18F] Florbetapir PET templates. Then landmark matching and thin-plate spline landmark-based deformation was performed. Finally, region-based standard uptake value ratios (SUVR) were calculated in predefined regions provided by the software:24 anterior cingulate, posterior cingulate, precuneus, inferior medial frontal, lateral temporal, and superior parietal cortex using whole cerebellum as a reference region. Global beta-amyloid burden was calculated as the mean of the six regions of interest.
Transcranial Doppler
Prior to cerebrovascular characterization, participants refrained from caffeine for 12 h, physical activity for 24 h and eating a large meal for 2 h.25 The study visit began between 7:30 and 9:00 a.m. for all participants. Participants first completed health history questionnaires and were classified as having low, moderate, or high cardiovascular risk according to American College of Sports Medicine criteria.26 The laboratory room for the experimental session was dimly lit, quiet, and temperature maintained between 22 and 24 ℃.27,28 External stimuli were kept to a minimum during the testing session.29
The left MCA was used for the transcranial Doppler (TCD) ultrasound with a 2-MHz probe (RobotoC2MD, Multigon Industries) placed over the temporal window and fixed in place using a robotic TCD headpiece. If the left MCA was not obtainable, then the right side was used.29 Once the optimal signal was identified, we began the imaging process for mean MCA velocity (MCAvmean). Individuals performing the TCD data collection were blinded to beta-amyloid status.
A finger plethysmograph (Finometer Pro, Finapres Medical Systems) was placed on the middle finger of the left hand supported at the level of the heart on an adjustable padded bedside table. The finger plethysmograph collected continuous measures for mean arterial pressure (MAP). End-tidal CO2 (ETCO2) was assessed using a nasal cannula and capnograph (BCI Capnocheck 9004). We used a 5-lead electrocardiogram for heart rate (HR). The 8-min average MAP, ETCO2, and MCAvmean for each condition (resting and moderate intensity) were used.
Resting protocol
Participants sat quietly on the recumbent stepper (NuStep, T5XR) to rest for 15 min. During the last 8 min of rest, baseline data for all variables were recorded.
Exercise protocol
After the 8-min resting data collection, the participant performed a single bout of exercise at moderate intensity using the recumbent stepper. Moderate intensity exercise was defined as 40%–60% of age-predicted HR reserve.26 Participants maintained a step rate of approximately 90 steps per minute.30
All participants began exercise at 40 W. The resistance was increased until the target HR range was reached. The participant then continued with the steady state HR for one continuous minute to ensure target HR could be maintained with exercise. Then data collection commenced while the participant exercised for 8 min in the target HR range. Data were sampled at 500 Hz using an analog to digital data acquisition board (National Instruments) and custom script written for MATLAB (v2015, Mathworks).
Vascular measures
MCAvmean, MAP, and ETCO2 were resampled at 10 Hz. We calculated CVR as the change in MCAvmean (cm/s) from rest to moderate intensity exercise. We reported MCAvmean, MAP, and ETCO2 at rest and exercise.
Statistical analyses
All statistical analyses were performed using R (version 3.2.431). Between-group differences (beta-amyloid “elevated,” “non-elevated”) were assessed using Welch's one-way ANOVA (due to different sample sizes and variance characteristics) or Chi-square tests without continuity correction as appropriate. We performed linear regression to explore relationships between our vascular, cognitive, and brain amyloid measures. When primary regression proved significant, we performed a subsequent regression including, age, American College of Sports Medicine cardiovascular risk classification, change in ETCO2 from resting to baseline, and change in MAP from resting to baseline to control for potential effects on cerebrovascular dynamics. We set α=0.05 to protect against Type I error and did not correct for multiple comparisons due to the exploratory nature of this study.
Results
We included 88 individuals meeting study criteria. The time between the PET scan and the TCD ultrasound study visit was 63.8 ± 61.6 days. Individuals were excluded from data analysis due to: (1) inability to obtain a quality signal for the MCA (n = 13) either at rest or during exercise, (2) significant artifact during moderate intensity exercise (n = 4), and (3) participant inability to complete the 8-min exercise bout (n = 1). No serious adverse events were reported during or following the exercise bout.
Of the remaining 70 individuals (see Table 1; 25 elevated, 45 non-elevated), there was no difference in age, sex, and cardiovascular risk (p > 0.09) across amyloid elevated and non-elevated groups. All individuals classified as elevated had global beta-amyloid burden > 1.1, previously identified as a threshold sensitive for moderate-to-frequent neuritic plaques24,32 No individuals classified as non-elevated had a global beta-amyloid burden > 1.1.
Table 1.
Baseline demographics.
| Non-elevated (n = 45) | Elevated (n = 25) | All participants (n = 70) | P | |
|---|---|---|---|---|
| Age, years | 70.1 [4.8] | 72.0 [4.9] | 70.8 [4.9] | 0.13 |
| Female, n [%] | 31 [68.9] | 11 [68.8] | 41 [68.9] | 0.85 |
| Education, years | 16.9 [2.7] | 16.6 [3.2] | 16.8 [2.9] | 0.70 |
| Cardiovascular risk M:H | 20:25 | 6:19 | 26:44 | 0.09 |
| Memory | 0.8 [1.0] | 0.2 [0.9] | 0.5 [1.0] | 0.01 |
| Executive Function | 0.3 [0.6] | 0.3[0.4] | 0.3 [0.6] | 0.99 |
| Processing Speed | 0.4 [0.6] | 0.0 [0.5] | 0.3 [0.6] | 0.01 |
| Language | −0.4 [0.7] | 0.1 [0.7] | −0.2 [0.7] | 0.02 |
| Attention | −0.1 [0.8] | −0.3 [0.6] | −0.2 [0.7] | 0.30 |
Note: Values are mean [standard deviation] unless otherwise noted. Moderate: High risk (M:H). Cognitive values are mean z-scores of tests in each domain.
Cerebrovascular regulation is sensitive to both ETCO2 and MAP. Therefore, we assessed for group differences in these measures (Table 2). Neither resting ETCO2 (F = 0.7, p = 0.41) nor moderate intensity exercise ETCO2 (F = 0.8, p = 0.37) were different between groups. Neither resting MAP (F = 0.7, p = 0.40) nor moderate intensity exercise MAP (F = 0.8, p = 0.36) were different between groups.
Table 2.
Rest and exercise measures for CVR, MAP, and ETCO2.
| Non-elevated (n = 45) | Elevated (n = 25) | All participants (n = 70) | P | |
|---|---|---|---|---|
| CVR (cm/s) | 7.2 [5.0] | 3.3 [3.7] | 5.8 [4.9] | 0.001 |
| MCAvmean at rest(cm/s) | 48.1 [8.7] | 46.8 [10.2] | 47.6 [9.2] | 0.59 |
| MCAvmean during mod. exercise (cm/s) | 55.3 [10.8] | 50.1 [10.0] | 53.4 [10.7] | 0.05 |
| MAP at rest (mmHg) | 74.5 [13.1] | 72.2 [9.9] | 73.7 [12.0] | 0.40 |
| MAP during mod. exercise (mmHg) | 105.9 [22.2] | 101.6 [17.1] | 104.4 [20.5] | 0.36 |
| Mean ETCO2 at rest (mmHg) | 33.0 [5.9] | 34.0 [3.7] | 33.4 [5.3] | 0.41 |
| Mean ETCO2 during mod. exercise (mmHg) HR at rest (bpm) | 37.2 [5.3] 66.4 [9.1] | 38.1 [2.9] 67.8 [9.5] | 37.5 [4.5] 67.0 [9.2] | 0.37 0.58 |
| HR during mod. | 108.3 [12.3] | 108.6 [9.3] | 108.4 [11.0] | 0.94 |
| exercise (bpm) |
Note: All measures mean [standard deviation].
CVR: cerebrovascular response; MCAvmean: mean velocity of middle cerebral artery; MAP: mean arterial pressure; ETCO2: end-tidal carbon dioxide; bpm: beats per minute.
Vascular measures
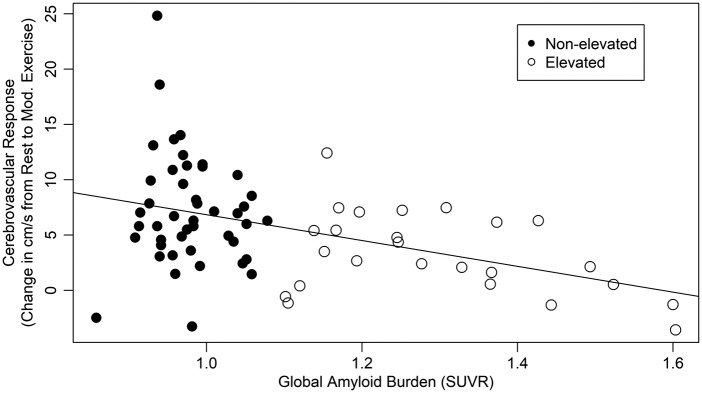
Our primary measure was CVR, measured as the change in MCAvmean between resting and moderate intensity exercise. First, we assessed group differences in resting MCAvmean. Elevated individuals did not have different resting MCAvmean than their non-elevated peers (F = 0.3, p = 0.59) and global beta-amyloid burden was not related to resting MCAvmean (b = −5.5 [95% CI −17.7, 6.8], p = 0.38). Next, we found that individuals with elevated beta-amyloid had a blunted (54% lower) CVR compared to those without elevated beta-amyloid (F = 13.5, p < 0.001). We then examined the relationship of CVR and beta-amyloid burden. Lower CVR was significantly associated with greater global beta-amyloid burden (b = −11.7 [95% CI −17.6, −5.7], p < 0.001; Figure 1). That is, each increase in SUVR of 0.1 units was associated with a 1.17 cm/s decline in CVR. This relationship held even when controlling for age, cardiovascular risk, change in ETCO2 and MAP from resting to baseline (b = −11.3 [95% CI −17.6, −4.9], p < 0.001).
Figure 1.
Cerebrovascular response was associated with global beta-amyloid burden.
Cognitive measures
Group differences were evident in the Memory (F = 6.4, p = 0.01), Processing Speed (F = 6.9, p = 0.01), and Language cognitive domains (F = 5.4, p = 0.02). Non-elevated individuals performed better than their elevated peers in the Processing Speed and Memory domains. Elevated individuals performed better in the Language domain. Executive Function and Attention were not different between groups (p ≥ 0.2).
CVR was associated only with Processing Speed (b = 0.03 [95% CI 0.002, 0.059], p = 0.001). This relationship was attenuated when controlling for age, cardiovascular risk, and change in ETCO2 and MAP (p = 0.12).
Global beta-amyloid burden was significantly related to Memory such that greater burden was associated with poorer performance (b = −1.73 [95% CI −3.03, −0.43], p = 0.009). This relationship remained significant even after controlling for age, cardiovascular risk, and change in ETCO2 and MAP (p = 0.047). Global beta-amyloid burden was also significantly related to Processing Speed such that greater burden was associated with poorer performance (b = −1.10 [95% CI −1.87, −0.34], p = 0.005). This relationship remained significant even after controlling for age, cardiovascular risk, and change in ETCO2 and MAP (p = 0.03). Finally, global beta-amyloid burden was significantly related to Language such that greater burden was associated with better performance (b = 1.14 [95% CI 0.17, 2.1], p = 0.02). This relationship remained significant even after controlling for age, cardiovascular risk, and change in ETCO2 and MAP (p = 0.02).
Discussion
The major finding from the present study was that the individuals in the elevated beta-amyloid group demonstrated 54% lower CVR compared to participants in the non-elevated group. These data are consistent with beta-amyloid-driven changes in cerebrovascular control seen in mice.2,3 Of importance, the two groups were not statistically different for resting MCAvmean, MAP, and ETCO2 and the change in MAP and ETCO2 from rest to exercise was not significantly different. This suggests that MCAvmean was not singularly driven by changes in MAP or ETCO2, which are known to directly influence cerebral blood flow. Our work is the first to show that CVR during exercise is negatively affected by the presence of greater beta-amyloid burden. This finding is consistent with published work in humans examining cerebrovascular regulation and beta-amyloid.7,33
In our cohort of cognitively normal older adults, we did not find differences in resting MCAvmean. However, prior work highlighted decreases in resting cerebral blood flow (arterial spin labeling) with higher beta-amyloid burden.34 The authors tested whether regional cerebral blood flow would be different between healthy control and three distinct diagnostic groups (early cognitive impairment, late mild cognitive impairment, and Alzheimer's dementia). The authors found that independent of diagnostic group, those with higher beta-amyloid had lower cerebral blood flow. Our study supports and extends prior work that increasing amyloid burden negatively interacts with cerebrovascular regulation. Specifically, we report CVR to exercise was blunted in those with elevated beta-amyloid.
The present findings complement previous work in animal model where the authors reported that mice (two to three months old) with a genetic disposition for higher levels of beta-amyloid precursor protein (APP) demonstrated disruption in cerebral autoregulation in response to pressure changes, whereas wild-type showed no disruption.2 Further, the APP + mice with higher levels of beta-amyloid showed the greatest disruption in cerebrovascular regulation. Although this present study did not specifically assess cerebrovascular autoregulation, we did examine changes in CVR with concomitant changes in MAP and ETCO2 from rest to moderate intensity exercise. Our findings in cognitively normal older adults demonstrate that a blunted CVR was observed in those with higher levels of global beta-amyloid and are consistent with beta-amyloid-driven changes in cerebrovascular control seen in mice.
We chose to use exercise for the experimental protocol.12 Exercise presents a physiologic challenge to the cerebrovascular system due to rapid increases in MAP, increased sympathetic activity, and greater cardiac output.10,11 Quantitative measurement of cerebral blood flow at rest provides valuable steady state information. However, examining the dynamic response, CVR, from rest to moderate intensity exercise can provide unique information regarding cerebrovascular control mechanisms29 especially in a well-characterized cohort. All of our participants were sedentary26 and were classified as moderate or high cardiac risk.26 Second, we characterized our study participants according to beta-amyloid burden, a hallmark pathology of Alzheimer's disease.1 A strength of our study was the inclusion of a well-defined cohort of participants with standard cognitive testing and PET imaging. With the ever-increasing burden of cerebrovascular disease and dementia, this well-characterized group is essential to ultimately identify clinical tools that might detect early changes in cerebrovascular health.
The early identification of cerebrovascular impairment would have great clinical and global implications35 especially if the onset of dementia could be delayed. For our secondary hypothesis, the data would suggest a possible association between CVR and speeded tasks. This is notable because many of the cognitive tasks, specifically speeded performance, benefit from improved cardiovascular health.36,37 It is perhaps not surprising that cognitive performance was worse in the elevated beta-amyloid group. Our findings are consistent with previous reports that have linked beta-amyloid burden to cognitive decline in cognitively normal older adults.38,39 Future work should consider a larger sample and longitudinal design to further elucidate any interaction or potential mediation effect between CVR, global amyloid burden, and cognitive function.7,33
Although exercise-induced brain blood flow response is less understood in older adults, we have provided novel findings to further support the interaction of beta-amyloid and cerebrovascular regulation in the growing body of literature. We acknowledge that the non-elevated beta-amyloid group had a slightly higher resting and exercise MAP, which could influence MCAvmean. However, we report no between-group differences for resting or change in MAP or ETCO2 in response to moderate intensity exercise. We cannot rule out that there may be other cardiovascular measures that could influence CVR that were not examined in this study such as stroke volume, carotid-intima thickness, or arterial stiffness. However, we did capture cardiac risk and report similar representation across the two groups for moderate and high cardiovascular risk. Future work should include a comprehensive cardiac and vascular profile to extend upon these initial findings.
The present study has several limitations that ought to be considered when interpreting the results. First, this study did not focus on the mechanisms that may influence CVR in those with elevated global beta-amyloid. There may be other factors that influence cerebrovascular function such as cardiac function, hypertension, and peripheral vascular dysfunction. As observed in Figure 1, there are non-elevated individuals who had a blunted CVR response. Future work should focus on additional vascular measures to better understand the relationship between beta-amyloid and vascular health in humans. Second, we did not use controlled methods for regulating changes in blood pressure or CO2 (i.e. CO2 inhalation) when measuring MCAvmean across all subjects. Rather, we assessed each individual during moderate intensity exercise in the participant's age-predicted HR range. This can influence individual responsiveness. However, we believe our methodology is a strength of the study as it presents more real-world physiologic challenge versus CO2 gas inhalation. We acknowledge that we were unable to measure changes in MCA diameter. The assumption of constant MCA diameter is important for MCAV to be used as a direct proxy for cerebral blood flow. Currently, there is a lack of agreement whether MCA diameter changes with exercise or not40,41 but if the MCA diameter does change it may be negligible in larger vessels such as the MCA.42,43 Additionally, we were unable to acquire T1-weighted MRI to support regional hypothesis testing or increase the power of our analyses.44,45 This study is a first of kind exploratory study and as such the findings should be interpreted accordingly.
Conclusion
The findings of the present study demonstrate that cognitively normal individuals with elevated cerebral beta-amyloid demonstrate a blunted CVR than those without elevated cerebral beta-amyloid. CVR to moderate intensity exercise may have the potential to serve as a biomarker for brain health. These results in humans corroborate prior animal data whereby beta-amyloid accumulation may negatively impact cerebrovascular function.
Acknowledgements
We would like to thank Torrie Cross (University of Kansas), Kelsy Schoen (University of Kansas Medical Center), Andrea Sander (University of Kansas Medical Center), and Dana Stumpff (University of Kansas Medical Center) with their help in data collection and Kayla Meyer for her assistance with recruitment.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Dr. Billinger) and by the American Heart Association Grant 16GRNT30450008 (Dr. Billinger). Dr. Sisante and Ms. Kwapiszeski were supported in part by T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Ms. Kwapiszeski was supported in part by the American Heart Association Student Scholarship in Cerebrovascular Disease and Stroke. This project was supported by the University of Kansas Alzheimer's Disease Center (P30 AG035982). Avid Radiopharmaceutical and Eli Lilly and Co provided a grant to support the florbetapir imaging procedure. This work received support from the Landon Center on Aging endowments. This project was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS Grant Number UL1TR000001. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Jason-Flor Sisante: study concept and design, drafting/revising manuscript, acquisition of data, statistical analysis.
Eric Vidoni: study concept and design, drafting/revising manuscript, analysis or interpretation of data, statistical analysis.
Kiersten Kirkendoll: study concept and design, acquisition of data, and manuscript revision.
Jaimie Ward: acquisition of data, analysis or interpretation of data, manuscript revision.
Yumei Liu: acquisition of data, analysis or interpretation of data, manuscript revision.
Sarah Kwapiszeski: study concept and design, acquisition of data, drafting manuscript.
Rebecca Maletsky: analysis or interpretation of data, contribution of vital tools, statistical analysis.
Jeff Burns: study concept and design, study supervision, obtaining of funding.
Sandra Billinger: study concept and design, drafting/revising manuscript, analysis or interpretation of data, study supervision, obtaining of funding.
References
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niwa K, Kazama K, Younkin L, et al. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circulat Physiol 2002; 283: H315–H323. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C, Zhang F, Niwa K, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 1999; 2: 157–161. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer's dementia. Cell Mol Neurobiol 2003; 23: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yew B, Nation DA, Alzheimer's Disease Neuroimaging I. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 2017; 140: 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman RF, Schneider AL, Zhou Y, et al. association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017; 317: 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangen KJ, Clark AL, Edmonds EC, et al. Cerebral blood flow and amyloid-beta interact to affect memory performance in cognitively normal older adults. Front Aging Neurosci 2017; 9: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dementia 2015; 11: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie L, Dolui S, Das SR, et al. A brain stress test: cerebral perfusion during memory encoding in mild cognitive impairment. Neuroimage Clin 2016; 11: 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol 2000; 61: 397–414. [DOI] [PubMed] [Google Scholar]
- 11.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med 2007; 37: 765–782. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JP, Ogoh S, Young CN, et al. Regulation of middle cerebral artery blood velocity during dynamic exercise in humans: influence of aging. J Appl Physiol 2008; 105: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breault C, Piper J, Joshi AD, et al. Correlation between two methods of florbetapir PET quantitative analysis. Am J Nucl Med Mol Imag 2017; 7: 84–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Harn NR, Hunt SL, Hill J, et al. Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med 2017; 42: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004; 18: 270–277. [PubMed] [Google Scholar]
- 16.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 17.Monsell SE, Dodge HH, Zhou XH, et al. Results from the NACC uniform data set neuropsychological battery crosswalk study. Alzheimer Dis Assoc Disord 2016; 30: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graves RS, Mahnken JD, Swerdlow RH, et al. Open-source, rapid reporting of dementia evaluations. J Registry Manag 2015; 42: 111–114. [PMC free article] [PubMed] [Google Scholar]
- 19.Shirk SD, Mitchell MB, Shaughnessy LW, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther 2011; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grober E, Buschke H, Crystal H, et al. Screening for dementia by memory testing. Neurology 1988; 38: 900–903. [DOI] [PubMed] [Google Scholar]
- 21.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology 2008; 71: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LIlly USA L. Amyvid Reader Training Program. 2014. Available at: amyvid.myregistrationp.com.
- 23.Burns JM, Johnson DK, Liebmann EP, et al. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement 2017; 13: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 2012; 11: 669–678. [DOI] [PubMed] [Google Scholar]
- 25.Tyndall AV, Davenport MH, Wilson BJ, et al. The brain-in-motion study: effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC Geriatr 2013; 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACSM. ACSM's guidelines for exercise testing and prescription, Philadephia, PA: Lippincott Williams & Wilkins, 2014, pp. 456. [Google Scholar]
- 27.Billinger SA, Sisante JV, Alqahtani AS, et al. Aerobic exercise improves measures of vascular health in diabetic peripheral neuropathy. Int J Neurosci 2016; 127: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billinger SA, Sisante JV, Mattlage AE, et al. The relationship of pro-inflammatory markers to vascular endothelial function after acute stroke. Int J Neurosci 2016; 127: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billinger SA, Craig JC, Kwapiszeski SJ, et al. Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. J Appl Physiol 2017; 122: 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billinger SA, Tseng BY, Kluding PM. Modified total-body recumbent stepper exercise test for assessing peak oxygen consumption in people with chronic stroke. Phys Ther 2008; 88: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing, 2011. [Google Scholar]
- 32.Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med 2012; 53: 378–384. [DOI] [PubMed] [Google Scholar]
- 33.Brickman AM, Guzman VA, Gonzalez-Castellon M, et al. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci Lett 2015; 592: 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattsson N, Tosun D, Insel PS, et al. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer's disease and mild cognitive impairment. Brain 2014; 137: 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malojcic B, Giannakopoulos P, Sorond FA, et al. Ultrasound and dynamic functional imaging in vascular cognitive impairment and Alzheimer's disease. BMC Med 2017; 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A 2004; 101: 3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature 1999; 400: 418–419. [DOI] [PubMed] [Google Scholar]
- 38.Farrell ME, Kennedy KM, Rodrigue KM, et al. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose-response relationship. JAMA Neurol 2017; 74: 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donohue MC, Sperling RA, Petersen R, et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017; 317: 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 2016; 594: 4073–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brothers RM, Zhang R. CrossTalk opposing view: the middle cerebral artery diameter does not change during alterations in arterial blood gases and blood pressure. J Physiol 2016; 594: 4077–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber P. Angiographic evaluation of internal carotid blood flow in patients with cerebrovascular disease. Radiol Clin Biol 1967; 36: 82–90. [PubMed] [Google Scholar]
- 43.Giller CA, Bowman G, Dyer H, et al. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 1993; 32: 737–741. discussion 41–42. [PubMed] [Google Scholar]
- 44.Brendel M, Delker A, Rotzer C, et al. Impact of partial volume effect correction on cerebral beta-amyloid imaging in APP-Swe mice using [(18)F]-florbetaben PET. Neuroimage 2014; 84: 843–853. [DOI] [PubMed] [Google Scholar]
- 45.Brendel M, Hogenauer M, Delker A, et al. Improved longitudinal [(18)F]-AV45 amyloid PET by white matter reference and VOI-based partial volume effect correction. Neuroimage 2015; 108: 450–459. [DOI] [PubMed] [Google Scholar]