Abstract
Background:
The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure (BP) guideline recommended lower BP thresholds for antihypertensive medication initiation and intensification compared to previous guidelines. We estimated the number of cardiovascular disease (CVD) events prevented and treatment-related serious adverse events incurred over ten years among US adults with hypertension by achieving 2017 ACC/AHA guideline-recommended BP goals compared with 1) current BP levels, 2) achieving 2003 Seventh Joint National Committee Report (JNC7) BP goals, and 3) achieving 2014 Eighth Joint National Committee panel member report (JNC8PM) BP goals.
Methods:
US Adults aged ≥45 years with an indication for BP treatment were grouped according to recommendations for antihypertensive drug therapy in the 2017 ACC/AHA guideline, JNC7, and JNC8PM. Population sizes were estimated from the 2011–2014 National Health and Nutrition Examination Surveys. Rates for fatal and non-fatal CVD events (stroke, coronary heart disease, or heart failure) were estimated from the REasons for Geographic And Racial Differences in Stroke study, weighted to the US population. CVD risk reductions with treatment to BP goals and risk for serious adverse events were obtained from meta-analyses of BP-lowering trials. CVD events prevented and treatment-related non-fatal serious adverse events over ten years were calculated. Uncertainty surrounding main data inputs was expressed in uncertainty ranges (URs).
Results:
Over ten years, achieving and maintaining 2017 ACC/AHA guideline goals compared with current BP levels, achieving JNC7 goals, or achieving JNC8PM goals could prevent 3.0 million (UR 1.1–5.1 million), 0.5 million (UR 0.2–0.7 million), or 1.4 million (UR 0.6–2.0 million) CVD events, respectively. Compared with current BP levels, achieving and maintaining 2017 goals could prevent 71.9 (UR 26.6–122.3) CVD events per 1,000 treated. Achieving 2017 guideline BP goals compared with current BP levels could also lead to nearly 3.3 million (UR 2.2–4.4 million) more serious adverse events over 10 years.
Conclusions:
Achieving and maintaining 2017 ACC/AHA BP goals could prevent a greater number of CVD events than by achieving JNC7 or JNC8PM BP goals but could also lead to more serious adverse events.
Keywords: guidelines, blood pressure, hypertension, cardiovascular disease, prevention
INTRODUCTION
The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure (BP) guideline recommended initiation of antihypertensive medication based on a combination of cardiovascular disease (CVD) risk and average BP level, and lowered BP treatment goals.1 It has been estimated that the application of the 2017 ACC/AHA BP guidelines would result in a recommendation for antihypertensive drug therapy in approximately 4.2 million additional US adults and would increase the number of adults with hypertension who have a BP above the recommended target of <130/80 mm Hg by about 7.9 million adults compared to the 2003 Seventh Joint National Committee Report (JNC7) guideline.1, 2
Intensive BP treatment to a systolic BP goal of 120 or 130 mmHg results in a larger reduction in CVD risk compared with treatment to 140 or 150 mmHg.3–5 For the same lowering of BP, absolute risk reduction is greater in patients with higher CVD risk.6 Thus, the 2017 ACC/AHA BP guideline recommendation of antihypertensive drug treatment based on a combination of BP levels and high CVD risk may be more efficient than prior recommendations in US BP guidelines. The risk-stratified approach may yield greater absolute CVD risk reduction for the same number of adults treated compared to what would be expected when treatment is based on BP levels only.7 However, high CVD risk patients might also be at increased risk for antihypertensive treatment-related serious adverse events (SAEs).
We estimated the potential population health impact, including benefits and harms, associated with achieving and maintaining 2017 ACC/AHA guideline-recommended BP goals in US adults aged ≥45 years with hypertension compared with maintaining current BP levels, achieving BP goals recommended in the 2003 JNC7 guideline or achieving 2014 Eighth Joint National Committee panel member (JNC8PM) report BP goals.1, 8, 9 The current analysis complements our previous estimates of the population recommended treatment initiation or intensification under the 2017 ACC/AHA BP guideline by projecting the potential number of CVD events that could be prevented if the 2017 ACC/AHA BP guideline-recommended BP goals were achieved and maintained.2 We compared these estimates with the number of CVD events expected with maintenance of current BP levels or with achievement of BP goals set by the two previous national guidelines.8, 9
METHODS
The data, analytic methods, and study materials will not be made publicly available to other researchers for purposes of reproducing the results or replicating the procedure. However, with review and approval, the information is available from The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study under established data sharing procedures. The National Health and Nutrition Examination Surveys (NHANES) are a publicly available data source.
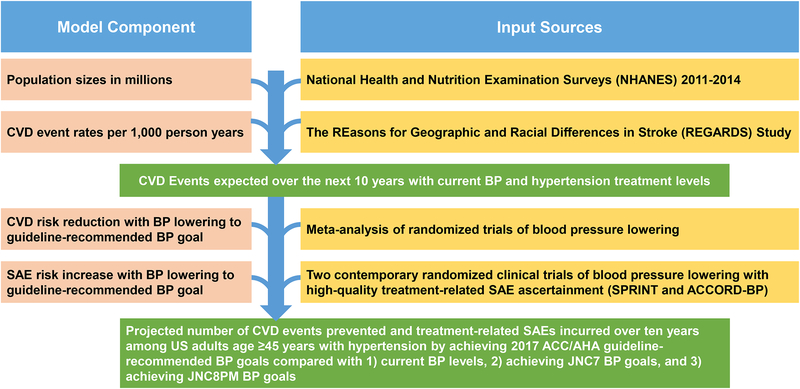
We defined mutually exclusive hypertension treatment groups based on recommendations in the 2017 ACC/AHA, JNC7, and JNC8PM guidelines using data on current antihypertensive medication use status, current BP level, age, and co-morbidities (Table S1, S2, and S3). For this simulation study, we estimated two inputs described in detail below: (1) 10-year CVD event rates in hypertension treatment groups, and (2) US adult population sizes for each group. Expected CVD risk reduction with BP lowering to goal was based on response to treatment to current and recommended BP levels in a meta-analysis of antihypertensive drug treatment randomized trials (Figure 1).3 Expected risk of treatment-related SAEs with BP lowering was based on treatment-related harms observed in patients treated to standard (i.e., treatment goal of SBP <140 mmHg) and intensive BP goals (i.e., treatment goal of SBP <120 mm Hg) using pooled data from the Systolic Blood Pressure Intervention Trial (SPRINT) and The Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial (ACCORD-BP).10, 11
Figure 1. Flowchart Illustrating Model Components And Their Data Sources Used To Calculate Projected Number Of Cardiovascular Disease Events Prevented And Treatment-Related Serious Adverse Events Incurred With Achieving And Maintaining The 2017 ACC/AHA, JNC7, And JNC8PM Guideline-Recommended SBP Goals Compared To Maintaining Current SBP Levels.
ACC: American College of Cardiology, AHA: American Heart Association, CVD: Cardiovascular disease, JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, JNC8PM: Eight Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, BP: Blood Pressure, SAE: Serious adverse event.
CVD event rates: The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study
The REGARDS study enrolled 30,239 black and white women and men age ≥45 years from all 48 contiguous US states and the District of Columbia between January 2003 and October 2007. We excluded participants missing baseline BP measurements (n=143), information on self-reported antihypertensive medication use (n=225), or variables needed to determine hypertension treatment group according to the 2017 ACC/AHA, JNC7 and JNC8PM guidelines (n=195).1, 8, 9 After excluding an additional 458 participants who lacked follow-up data, 29,218 participants were available for inclusion in our analyses (Figure S1). REGARDS participants were followed from baseline through the occurrence of a CVD event, death, loss-to-follow-up, or December 31, 2014, whichever occurred first. CVD events included stroke (fatal and nonfatal), coronary heart disease (fatal and nonfatal myocardial infarction or coronary heart disease death), or heart failure hospitalization (fatal or non-fatal). Event adjudication procedures have been described and are provided in the supplement (Table S4).12–16 We chose the REGARDS study as the sole source for blood pressure-related CVD event rates because it is one of the largest, most contemporary population-based samples of US adults with rigorously adjudicated CVD events. Additionally, the CVD event rates in the REGARDS study are likely to be more generalizable than those obtained in randomized trials.
Hypertension treatment group population sizes: National Health and Nutrition Examination Surveys (NHANES)
To attain sufficient sample size, we pooled NHANES surveys conducted between 2011 and 2014. Due to the REGARDS study age range, the NHANES analysis was restricted to participants ≥45 years of age. We included NHANES participants with three systolic and diastolic BP measurements obtained during the study visit (n=5,728). Participants missing data on variables needed to determine hypertension treatment group according to the 2017 ACC/AHA, JNC7 and JNC8PM guidelines were excluded (n=335).1, 8, 9 After these exclusions, data from 5,393 participants were available for analysis (Figure S2).
BP measurements in REGARDS and NHANES
For REGARDS study participants, BP was measured two times, in the seated position, after 5 minutes of rest, at least 30 seconds apart, by a trained health professional during in-home examinations using an aneroid sphygmomanometer and an appropriately sized cuff. The mean of the two measurements defined REGARDS participants’ BP. For NHANES participants, BP was measured three times in the seated position, after five minutes of rest, 30 seconds apart, by a trained physician using a mercury sphygmomanometer with an appropriately sized cuff. The mean of the three measurements defined BP in NHANES.
Benefits and risks of BP lowering
We estimated CVD risk reduction associated with achieving recommended versus current systolic BP levels using the hazard ratios estimated in a network meta-analysis of 42 randomized BP lowering trials including 144,220 participants (Table S5).3 Treatment benefits for the population with isolated diastolic hypertension or with higher diastolic BP hypertension stage than systolic BP stage were analyzed in sensitivity analyses that categorized adults into hypertension treatment groups based on the higher hypertension stage, considering of both diastolic or systolic BP (Table S6). We estimated risks for antihypertensive treatment-related SAEs using a weighted average of SAE risks observed in the SPRINT and ACCORD-BP (Table S7).10, 11
Statistical Analysis
We calculated characteristics of REGARDS and NHANES 2011–2014 study participants overall and among those taking and not taking antihypertensive medication (Table S8 and S9). We calculated population sizes from NHANES and the 10-year CVD event rates from the sampling-weighted REGARDS population for each hypertension treatment group while accounting for the competing risk of all-cause mortality (Tables S1 and S2).17 Because the REGARDS study only enrolled black and white participants and over-sampled blacks, we weighted the REGARDS study cohort to match the US adult population sizes by age, sex, and black/non-black race groups. Specifically, statistical weights were calculated for population subgroups defined by age, sex and race by dividing the percentage of US adults (estimated from NHANES) over the percentage of REGARDS study participants that belong to each subgroup. Analyses incorporated sampling weights using STATA V14 (Stata Corporation, College Station, TX). The 95% confidence intervals for the 10-year risk of CVD events were calculated as described by Fine and Gray.17 The 95% confidence intervals of each NHANES hypertension treatment group were calculated using Taylor-series variance estimation.18 Analyses incorporated sampling weights using STATA V14 (Stata Corporation, College Station, TX).
We multiplied the observed REGARDS 10-year CVD event rates upper and lower bound 95% confidence limits by hypertension treatment group size to project the number of CVD events expected over the next 10 years if current BP levels were to be sustained. Next, we multiplied this expected number of CVD events by the hazard ratio corresponding to current systolic BP and guideline-specific recommended BP goals (Table S4) to project population benefit: the number of CVD events prevented by achieving and maintaining the 2017 ACC/AHA or JNC7 guideline or JNC8PM BP goals for ten years. Treatment efficiency was calculated by dividing the projected number of events prevented by the number of adults treated, multiplied by 1,000. To quantify uncertainty, analysis-of-extremes sensitivity analyses were performed in which the number of CVD events prevented and treatment efficiency was re-calculated using the upper and lower 95% confidence bounds of both treatment effect size and REGARDS ten-year CVD event rates.19–21 For population health benefit and treatment efficiency estimates, uncertainty ranges (UR) represent the lower and upper bounds from the analysis-of-extremes sensitivity analysis.
To project population health harms (i.e., number of treatment-related SAEs) expected over 10 years associated with initiation or intensification of antihypertensive medication needed to achieve and maintain guideline-recommended BP goals compared with maintaining current BP levels, we multiplied the pooled 10-year risk of treatment-related SAEs in the standard treatment arms of SPRINT and ACCORD-BP (i.e., those treated to SBP <140 mmHg) to hypertension treatment groups when the guideline-recommended BP goals were <140 or 150 mmHg (Table S7).10, 11 For hypertension treatment groups with guideline-recommended BP goals of <130 mmHg, we multiplied the pooled intensive treatment arm SAE risk by the population sizes. Definitions of treatment-related SAEs in SPRINT and ACCORD-BP are provided in the supplement. SAEs included hypotension, syncope, bradycardia, electrolyte abnormalities, injurious falls, and acute kidney injury among others (Table S10).
Subgroup and Sensitivity Analyses
For sub-group analyses, NHANES-based hypertension treatment group size and REGARDS baseline CVD event rate estimates were generated for subgroups defined by sex, race/ethnicity (i.e., white, non-Hispanic black, Hispanic), age (< 65 and ≥65 years), chronic kidney disease (CKD) status defined by a self-report of being on dialysis, eGFR <60 ml/min/1.73 m2 or albumin to creatinine ratio of ≥30 mg/g, diabetes status, clinical CVD status, and age ≥65 years with “robust” health status (based on walking speed, prior falls, and mobility status; see Supplement). Because lower BP targets may be difficult to achieve in clinical practice settings, we repeated all the analyses assuming that only 75%, which reflects adherence rates observed in BP-lowering clinical trials22, of the treatment groups recommended a SBP goal of <130 mm Hg would reach this goal compared to 100% reaching the SBP goals of <140 or <150 mm Hg.
Main effectiveness estimates reflect incomplete medication adherence in the trials included in the network meta-analysis. The lower bounds of our uncertainty ranges imply an effect size reflecting medication adherence as low as 27% (Table S11).22
Data management was conducted using SAS version 9.4 (SAS Institute, Cary, NC) and data analysis using Stata V14 (Stata Corporation, College Station, TX) and Microsoft Excel. The REGARDS study and NHANES were approved by the Institutional Review Boards governing research in human subjects, and all participants provided written informed consent.
RESULTS
A total of 17.9, 14.4, and 9.6 million US adults age ≥ 45 years are recommended antihypertensive medication initiation by the 2017 ACC/AHA BP guideline, JNC7, and the JNC8PM, respectively. In addition, a total of 24.0, 18.8, and 11.4 million currently treated US adults age ≥ 45 years are recommended antihypertensive medication intensification by the 2017 ACC/AHA BP guideline, JNC7, and the JNC8PM, respectively.
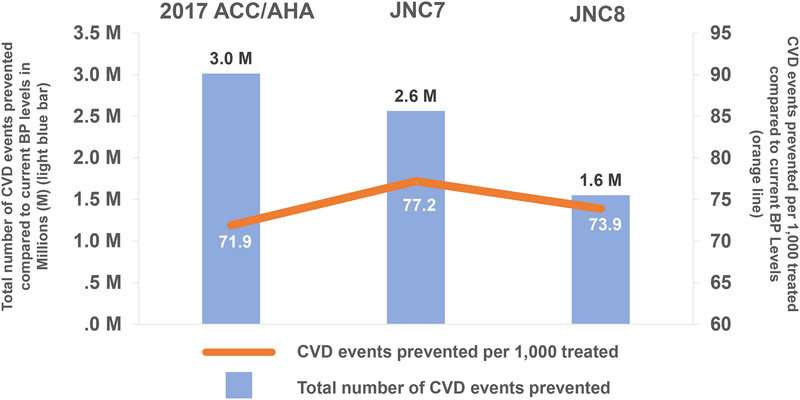
Compared with maintaining current BP levels, achieving the 2017 ACC/AHA guideline-recommended BP goals in US adults with hypertension could prevent 3.0 million CVD events over ten years of treatment (uncertainty-range [UR], 1.1–5.1) (Figure 2). Of these CVD events prevented, 83% resulted from treatment in adults with a current BP ≥140/90 mmHg; 35% of all CVD events prevented would be in those initiating antihypertensive treatment and 65% in those intensifying current antihypertensive treatment. Achieving 2017 ACC/AHA guideline BP goals would likely prevent 0.5 million (UR 0.2–0.7) and 1.4 million (UR 0.7–2.0) more CVD events compared to achieving the JNC7- or JNC8PM-recommended BP goals, respectively (Table 1 and Table 2). Achieving 2017 ACC/AHA guideline BP goals could also lead to an increase in treatment-related SAEs: 3.3 million (UR 2.2–4.4) more SAEs compared with maintaining current BP levels and 1.2 million (UR 0.8–1.6) and 2.4 million (UR 1.7–3.2) and more compared to achieving the JNC7- or JNC8PM-recommended BP goals (Figure 3 and Figure 4, Table S12 and S13).
Figure 2. Overall Projected Number of Cardiovascular Disease Events Prevented and Events Prevented Per 1,000 Treated US Adults by Achieving and Maintaining the 2017 ACC/AHA, JNC7, and JNC8PM Guideline-Recommended SBP Goals Compared to Maintaining Current SBP Levels.
ACC: American College of Cardiology, AHA: American Heart Association, CVD: Cardiovascular disease, JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, JNC8PM: Eight Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, SBP: Systolic Blood Pressure.
Table 1.
Projected number of US adults ≥ 45 years recommended and not recommended pharmacological antihypertensive treatment, CVD events expected under current SBP levels, and CVD potentially prevented in the next ten years by achieving and maintaining 2017 ACC/AHA and JNC7 Guideline-Recommended SBP Goals.
| US Adults, millions (95% CI) |
CVD events expected (95% CI) with current SBP levels, millions | Projected CVD events prevented (UR) with achieving guideline-recommended SBP goals, millions | |||
|---|---|---|---|---|---|
| JNC7 | 2017 ACC/AHA | Difference (UR) |
|||
| Currently not taking antihypertensive medication | |||||
| SBP <130 mm Hg | |||||
| Antihypertensive medication not recommended by 2017 ACC/AHA or JNC7 | 51.3 (44.5–58.1) |
3.6 (3.2–4.1) |
- | - | - |
| SBP 130 to <140 mm Hg | |||||
| Antihypertensive medication not recommended by 2017 ACC/AHA or JNC7 | 5.2 (3.6–6.7) |
0.2 (0.1–0.3) |
- | - | - |
| Antihypertensive medication recommended by the 2017 ACC/AHA but not by JNC7 | 3.5 (2.4–4.6) |
0.5 (0.4–0.6) |
- | 0.1 (0.0–0.2) |
0.1 (0.0–0.2) |
| Antihypertensive medication recommended by the 2017 ACC/AHA and by JNC7 | 3.0 (1.6–4.4) |
0.6 (0.5–0.8) |
0.1 (0.0–0.2) |
0.1 (0.0–0.2) |
0 |
| SBP ≥140 mm Hg | |||||
| Antihypertensive medication recommended by the 2017 ACC/AHA and by JNC7 | 11.4 (7.2–15.6) |
2.2 (1.5–3.0) |
0.8 (0.2–1.4) |
0.9 (0.3–1.6) |
0.1 (0.1–0.2) |
| Total | 74.3 (59.3–89.4) |
7.2 (5.9–8.7) |
0.8 (0.2–1.6) |
1.0 (0.3–1.9) |
0.2 (0.1–0.3) |
| Currently taking antihypertensive medication | |||||
| SBP <130 mm Hg | |||||
| Treatment intensification not recommended by the 2017 ACC/AHA or JNC7 | 24.7 (20.9–28.5) |
3.9 (3.6–4.1) |
- | - | - |
| SBP 130 to <140 mm Hg | |||||
| Treatment intensification recommended by the 2017 ACC/AHA but not JNC7 | 5.2 (3.8–6.6) |
0.7 (0.6–0.9) |
- | 0.1 (0.0–0.2) |
0.1 (0.0–0.2) |
| Treatment intensification recommended by the 2017 ACC/AHA and JNC7 | 5.0 (3.9–6.1) |
1.5 (1.3–1.7) |
0.2 (0.0–0.5) |
0.2 (0.0–0.5) |
0 |
| SBP ≥140 mm Hg | |||||
| Treatment intensification recommended by the 2017 ACC/AHA and by JNC7 | 13.8 (9.3–18.3) |
3.7 (3.0–4.6) |
1.5 (0.7–2.4) |
1.6 (0.8–2.5) |
0.1 (0.1–0.1) |
| Total | 48.7 (37.9–59.5) |
9.8 (8.5–11.3) |
1.7 (0.7–2.8) |
2.0 (0.8–3.2) |
0.2 (0.1–0.4) |
| Overall (Taking and not taking Antihypertensive Medication) | |||||
| Total | 123.1 (97.2–148.9) |
16.9 (14.3–19.5) |
2.6 (0.9–4.4) |
3.0 (1.1–5.1) |
0.5 (0.2–0.7) |
ACC: American College of Cardiology; AHA: American Heart Association; CI: confidence interval; CVD: cardiovascular disease; JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SBP: systolic blood pressure. In this table, recommendations for antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline but not JNC7 include adults without diabetes or CKD (1) age ≥65 years with SBP 130–139 mm Hg and, or (2) with SBP/DBP 130–139/80–89 mm Hg with a history of CVD or a 10-year predicted CVD risk ≥10% on the Pooled Cohort risk equations. Recommendations for antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline and JNC7 include (1) SBP/DBP 130–139/80–89 mm Hg with diabetes or CKD, and (2) SBP/DBP ≥140/80 mm Hg. Recommendation for intensification of antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline but not by JNC7 guideline includes SBP 130–139 mm Hg without diabetes or CKD. Recommendations for intensification of antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline and JNC7 include (1) SBP 130 to <140 mm Hg with diabetes or CKD, and (2) SBP ≥140 mm Hg.
UR: Uncertainty range. The uncertainty range represents the upper and lower bound from the analysis of extremes sensitivity analysis where the number of cardiovascular disease events prevented is recalculated using the upper and lower confidence bounds of both treatment effect size magnitude in the trials meta-analysis3 and REGARDS ten-year cardiovascular event rate.19–21 The lower bound is calculated using the upper bound of the hazard ratio and the lower bound of the REGARDS ten-year cardiovascular event rate. The upper bound is calculated using the lower bound of the hazard ratio and the upper bound of the REGARDS ten-year cardiovascular event rate.
Table 2.
Projected number of US adults ≥ 45 years recommended and not recommended pharmacological antihypertensive treatment, CVD events expected under current SBP levels, and projected CVD adverted in the next 10 years in adults by Achieving and Maintaining 2017 ACC/AHA and JNC8PM Guideline-Recommended SBP Goals.
| US Adults, millions (95% CI) |
CVD events expected with current SBP levels, millions, (95% CI) | Projected CVD events prevented with achieving guideline-recommended SBP goals, millions (UR) | |||
|---|---|---|---|---|---|
| JNC8PM (UR) |
2017 ACC/AHA (UR) |
Difference (UR) |
|||
| Currently not taking antihypertensive medication | |||||
| SBP <130 mm Hg | 51.3 (44.5–58.1) |
3.6 (3.4–3.9) |
- | - | - |
| SBP 130 to <140 mm Hg | |||||
| Antihypertensive medication not recommended by 2017 ACC/AHA or JNCPM | 5.2 (3.6–6.7) |
0.2 (0.1–0.3) |
- | - | - |
| Antihypertensive medication recommended by the 2017 ACC/AHA but not by JNC8PM | 6.5 (4.6–8.4) |
1.1 (0.9–1.3) |
- | 0.2 (0.0–0.3) |
0.2 (0.0–0.3) |
| SBP 140 to <150 mm Hg | |||||
| Antihypertensive medication recommended by the 2017 ACC/AHA and by JNC8PM | 4.5 (3.3–5.7) |
0.7 (0.5–1.0) |
0.1 (0.0–0.3) |
0.2 (0.1–0.4) |
0.1 (0.1–0.1) |
| Antihypertensive medication recommended by the 2017 ACC/AHA but not by JNC8PM | 1.8 (1.0–2.6) |
0.3 (0.2–0.5) |
- | 0.1 (0.0–0.2) |
0.1 (0.0–0.2) |
| SBP ≥ 150 mm Hg | |||||
| Antihypertensive medication recommended by the 2017 ACC/AHA and by JNC8PM | 5.1 (2.9–7.3) |
1.0 (0.7–1.5) |
0.4 (0.1–0.8) |
0.5 (0.2–0.9) |
0.2 (0.1–0.2) |
| Total | 74.3 (59.9–88.8) |
7.1 (5.9–8.5) |
0.5 (0.1–1.1) |
1.0 (0.3–1.9) |
0.5 (0.2–0.8) |
| Currently taking antihypertensive medication | |||||
| SBP <130 mm Hg | 24.7 (20.9–28.5) |
3.9 (3.6–4.1) |
- | - | - |
| SBP 130 to <140 mm Hg | |||||
| Treatment intensification recommended by the 2017 ACC/AHA but not JNC8PM | 10.2 (8.3–12.2) |
2.1 (1.8–2.4) |
- | 0.3 (0.0–0.6) |
0.3 (0.0–0.6) |
| SBP 140 to <150 mm Hg | |||||
| Treatment intensification recommended by the 2017 ACC/AHA and JNC8PM | 3.9 (2.9–4.9) |
1.0 (0.8–1.2) |
0.2 (0.0–0.4) |
0.3 (0.1–0.5) |
0.1 (0.1–0.1) |
| Treatment intensification recommended by the 2017 ACC/AHA but not JNC8PM | 2.4 (1.3–3.4) |
0.4 (0.3–0.6) |
- | 0.1 (0.0–0.2) |
0.1 (0.0–0.2) |
| SBP ≥150 mm Hg | |||||
| Treatment intensification recommended by the 2017 ACC/AHA and JNC8PM | 7.5 (5.1–9.9) |
2.3 (1.8–2.8) |
0.9 (0.3–1.5) |
1.2 (0.6–1.8) |
0.3 (0.3–0.2) |
| Total | 48.7 (38.5–58.9) |
9.7 (8.4–11.0) |
1.0 (0.3–1.9) |
2.0 (0.8–3.2) |
0.9 (0.5–1.2) |
| Overall (Taking and not taking Antihypertensive Medication) | |||||
| Total | 123.1 (98.4–147.7) |
16.9 (14.3–19.5) |
1.6 (0.5–2.9) |
3.0 (1.1–5.1) |
1.4 (0.7–2.0) |
ACC: American College of Cardiology; AHA: American Heart Association; CI: confidence interval; CVD: cardiovascular disease; JNC8PM: Eight Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SBP: systolic blood pressure. In this table, recommendations for antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline but not JNC7 include adults without diabetes or CKD (1) age ≥65 years with SBP 130–139 mm Hg and, or (2) with SBP/DBP 130–139/80–89 mm Hg with a history of CVD or a 10-year predicted CVD risk ≥10% on the Pooled Cohort risk equations. Recommendations for antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline and JNC7 include (1) SBP/DBP 130–139/80–89 mm Hg with diabetes or CKD, and (2) SBP/DBP ≥140/80 mm Hg. Recommendation for intensification of antihypertensive medication by the 2017 ACC/AHA high blood pressure guideline but not by JNC7 includes SBP 130–139 mm Hg without diabetes or CKD. Recommendations for intensification of antihypertensive medication by the 2017 ACC high blood pressure guideline and JNC7 include (1) SBP 130 to <140 mm Hg with diabetes or CKD, and (2) SBP ≥140 mm Hg.
UR: Uncertainty rage. The uncertainty range represents the upper and lower bound from the analysis of extremes sensitivity analysis where the number of cardiovascular disease events prevented is recalculated using the upper and lower confidence bounds of both treatment effect size magnitude in the trials meta-analysis3 and REGARDS ten-year cardiovascular event rate.19–21 The lower bound is calculated using the upper bound of the hazard ratio and the lower bound of the REGARDS ten-year cardiovascular event rate. The upper bound is calculated using the lower bound of the hazard ratio and the upper bound of the REGARDS ten-year cardiovascular event rate.
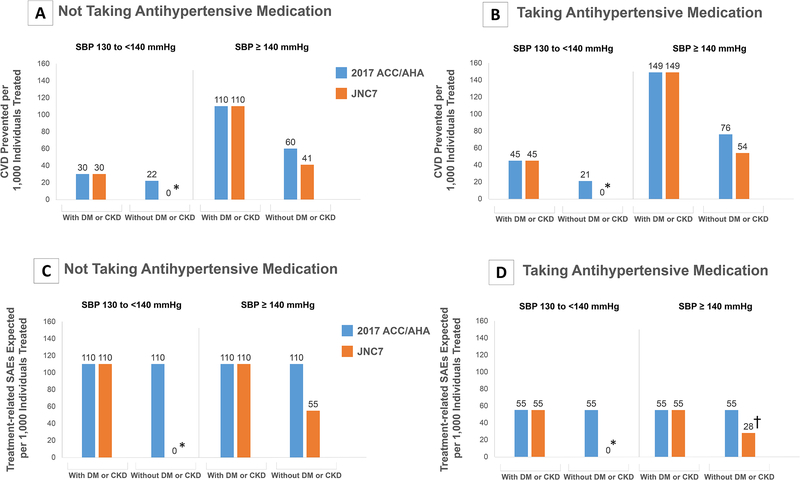
Figure 3. Cardiovascular Disease Events Prevented and Treatment-Related SAEs Expected Per 1,000 Treated by Achieving and Maintaining 2017 ACC/AHA and JNC7 Guideline-Recommended SBP Goals Compared to Maintaining Current SBP Levels According to Treatment Groups.
ACC: American College of Cardiology, AHA: American Heart Association, CVD: Cardiovascular disease, DM: Diabetes Mellitus, CKD: Chronic Kidney Disease, JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, JNC8PM: Eight Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, SBP: Systolic Blood Pressure, SAE: Serious Adverse Event.
Panels A and B show the number of cardiovascular disease events prevented per 1,000 individuals treated with achieving guideline-recommended blood pressure goals compared to current blood pressure levels for the 2017 ACC/AHA and the JNC7 guidelines within guideline-recommended treatment groups among those currently taking and not taking antihypertensive medication.
Panels C and D show the number of treatment-related SAEs expected with achieving guideline-recommended blood pressure goals compared to current blood pressure levels for the 2017 ACC/AHA and the JNC7 guidelines within guideline-recommended treatment groups among those currently taking and not taking antihypertensive medication.
The sub-groups presented represent different treatment target recommendations in the different guidelines. Cardiovascular disease events included stroke (fatal and nonfatal), coronary heart disease (fatal and nonfatal myocardial infarction or coronary heart disease death), or heart failure (fatal or non-fatal).
*Indicates a hypertension treatment group in which antihypertensive medication initiation of intensification is not recommended.
† Indicates treated uncontrolled groups where antihypertensive medication intensification is recommended. Due to lack of clinical-trial based evidence on incremental SAE risk in these groups, we assumed an additional SAE risk midway between the SAE risk with intensification to an intensive goal (<130/80 mmHg) and that of intensification to a <140/90 mmHg goal in treatment-naïve patients.
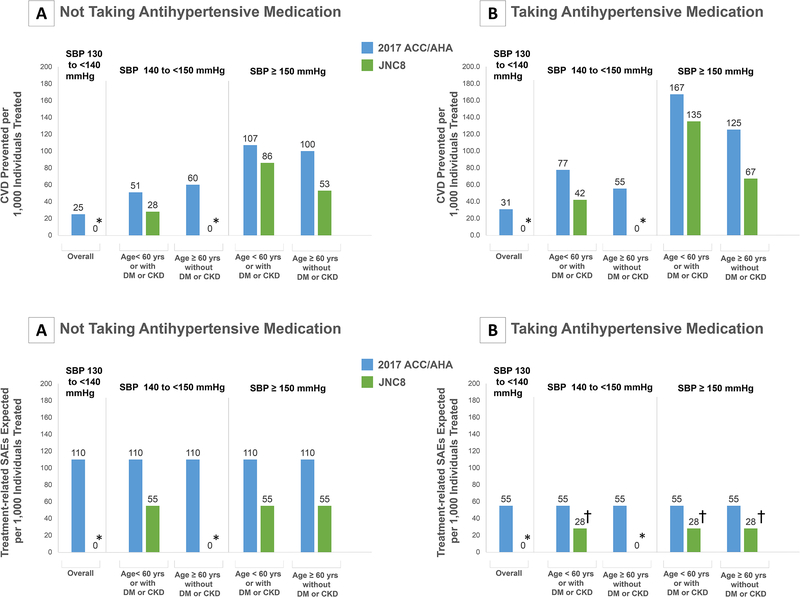
Figure 4. Cardiovascular Disease Events Prevented and Treatment-Related SAEs Expected Per 1,000 Treated by Achieving and Maintaining 2017 ACC/AHA and JNC8PM Guideline-Recommended SBP Goals Compared to Maintaining Current SBP Levels According to Hypertension Treatment Groups.
ACC: American College of Cardiology, AHA: American Heart Association, CVD: Cardiovascular disease, DM: Diabetes Mellitus, CKD: Chronic Kidney Disease, JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, JNC8PM: Eight Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, SBP: Systolic Blood Pressure.
Panels A and B show the number of cardiovascular disease events prevented per 1,000 individuals treated with achieving guideline-recommended blood pressure goals compared to current blood pressure levels for the 2017 ACC/AHA and the JNC8PM guidelines within guideline-recommended treatment groups among those currently taking and not taking antihypertensive medication.
The sub-groups presented represent different treatment target recommendations in the different guidelines.
Cardiovascular disease events included stroke (fatal and nonfatal), coronary heart disease (fatal and nonfatal myocardial infarction or coronary heart disease death), or heart failure (fatal or non-fatal).
*Indicates a hypertension treatment group in which antihypertensive medication initiation of intensification is not recommended.
† Indicates treated uncontrolled groups where antihypertensive medication intensification is recommended. Due to lack of clinical-trial based evidence on incremental SAE risk in these groups, we assumed an additional SAE risk midway between the SAE risk with intensification to an intensive goal (<130/80 mmHg) and that of intensification to a <140/90 mmHg goal in treatment-naïve patients.
Achieving and maintaining the 2017 ACC/AHA guideline goals for ten years could prevent 71.9 CVD events per 1,000 treated (UR 26.6–122.3) treated compared to maintain current BP levels (Figure 2). Compared to achieving JNC7 BP goals, achieving the 2017 guideline BP goals would be more efficient in patients with a current BP ≥140/90 mmHg and without diabetes or CKD (Figure 3). Achieving the 2017 guideline BP goals would be more efficient compared to achieving JNC8PM goals in all patients (Figure 4). Overall, treatment would also be more efficient in those intensifying current treatment compared with those initiating treatment.
Achieving the 2017 ACC/AHA guideline-recommended BP goals could prevent more CVD events than achieving JNC8PM goals in every sub-group or achieving JNC7 goals in every sub-group with the exception of people with diabetes and CKD (i.e., groups in which BP treatment targets are identical in the JNC7 and 2017 guidelines) (Table 3).
Table 3.
Comparison of the projected number of CVD events prevented in the next ten years by achieving and maintaining 2017 ACC/AHA and JNC8PM guideline-recommended SBP Goals compared to current SBP levels, overall and within subgroups.
| 2017 ACC/AHA | JNC7 | JNC8PM Panel Member Report | ||||
|---|---|---|---|---|---|---|
| Total CVD events prevented (millions, UR) | CVD events prevented per 1,000 treated (UR) | Total CVD events prevented (millions, UR) | CVD events prevented per 1,000 treated (UR) | Total CVD events prevented (millions, UR) | CVD events prevented per 1,000 treated (UR) | |
| Overall | 3.0 (1.1–5.1) |
71.9 (27.0–122.3) |
2.6 (0.9–4.4) |
77.3 (28.2–134.1) |
1.6 (0.5–2.9) |
73.9 (18.0–140.5) |
| Sex | ||||||
| Females | 1.5 (0.7–2.4) |
74.8 (35.0–117.6) |
1.3 (0.1–0.3) |
83.7 (37.2–133.0) |
0.8 (0.2–1.4) |
52.0 (8.2–113.7) |
| Males | 1.5 (0.7–2.3) |
80.1 (37.7–124.0) |
1.3 (0.6–2.0) |
86.7 (39.8–137.0) |
0.8 (0.3–1.3) |
70.9 (17.5–134.6) |
| Race | ||||||
| White | 2.1 (1.0–3.2) |
76.8 (37.4–115.5) |
1.8 (0.2–0.4) |
85.9 (39.9–132.2) |
1.0 (0.4–1.8) |
81.6 (28.5–138.7) |
| Black | 0.4 (0.2–0.7) |
75.2 (33.6–131.1) |
0.4 (0.2–0.7) |
81.0 (36.5–140.4) |
0.3 (0.1–0.5) |
82.7 (27.6–161.1) |
| Hispanic | 0.3 (0.1–0.4) |
82.3 (38.7–124.0) |
0.3 (0.1–0.4) |
86.7 (38.6–134.1) |
0.2 (0.1–0.3) |
82.4 (29.8–139.1) |
| Age, years | ||||||
| <65 | 1.0 (0.4–1.6) |
51.4 (19.9–85.6) |
0.8 (0.3–1.4) |
55.9 (20.3–95.5) |
0.5 (0.2–0.9) |
51.3 (17.3–94.4) |
| ≥65 | 2.0 (1.0–3.0) |
99.7 (48.3–150.7) |
1.8 (0.8–2.8) |
108.5 (51.1–167.0) |
1.1 (0.4–1.8) |
116.4 (40.8–197.1) |
| ≥ 65 and robust† | 1.5 (0.8–2.3) |
104.1 (56.0–157.5) |
1.4 (0.7–2.1) |
109.0 (55.1–167.3) |
0.7 (0.1–1.4) |
73.4 (8.0–153) |
| High-risk groups | ||||||
| Chronic kidney disease | 1.7 (0.9–2.5) |
126.6 (68.9–183.2) |
1.7 (0.9–2.5) |
126.6 (68.9–183.2) |
1.0 (0.5–1.6) |
121.0 (53.3–188.6) |
| Diabetes | 1.1 (0.4–1.7) |
80.8 (34.3–129.4) |
1.1 (0.4–1.7) |
80.8 (34.3–129.4) |
0.5 (0.2–0.9) |
102.8 (39.1–174.9) |
| Clinical CVD | 0.9 (0.4–1.4) |
126.5 (60.0–192.5) |
0.8 (0.4–1.3) |
139.2 (66.1–210.4) |
0.5 (0.2–0.8) |
149.7 (61.2–240.5) |
ACC: American College of Cardiology; AHA: American Heart Association; CVD: cardiovascular disease; JNC7: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SBP: systolic blood pressure; UR: uncertainty range.
Robust only is defined in NHANES by defined by walking at least 0.8 m/s in the standard 20-foot walk test, a threshold based on the prior studies recommending this cut-point and because it aligned with more comprehensive assessments of frailty and discriminated between survival times. Prior research has shown that among the traditional components used to assess frailty, slow gait speed has the strongest prognostic ability.22–25 Robust only is defined in REGARDS as without a history of falls or impaired mobility at the baseline visit.
Sensitivity Analyses
Results were similar when high diastolic BP was incorporated into assigning BP lowering effects (Table S14 and S15). In a sensitivity analysis where we assumed only 75% of those with recommended SBP goals of <130 mm Hg would reach this target (where recommended in the 2017 ACC/AHA guideline or JNC7 report) and 100% of those with SBP goals of <140 or <150 mm Hg would reach their recommended target (where recommended in the JNC7 or JNC8PM guidelines), the number of projected CVD events prevented was still larger with partial implementation of the 2017 ACC/AHA BP guideline (2.7 million UR 1.0–4.6) compared with complete implementation of the JNC7 guideline (2.4 million UR 0.8–4.1) and the JNC8PM (1.6 million UR 0.5–2.9) and remained efficient at 64.8 (UR 23.6–111.9) CVD events per 1,000 treated.
DISCUSSION
Implementing, achieving, and maintaining the 2017 ACC/AHA guideline-recommended BP goals would likely prevent about 3.0 million CVD events over ten years compared with sustaining current BP levels in US adults age ≥45 years with hypertension. This represents a larger population health impact than would be expected by achieving BP goals recommended in the JNC7 or JNC8PM guidelines. All population subgroups would benefit by achieving the 2017 guideline goals. The majority (83%) of the CVD events that would be prevented by achieving 2017 guideline goals would accrue in patients with a current BP ≥140/90 mmHg, a level of BP for which treatment with antihypertensive drug therapy has been recommended for more than 25 years.8, 23, 24
The current analysis suggests that achieving and maintaining 2017 ACC/AHA BP guideline goals would be efficient, preventing 71.9 CVD events per 1,000 treated adults over ten years compared with maintaining current BP levels. Treatment was also efficient among those recommended drug treatment initiation or intensification. This finding is important because some investigators have voiced concern that the 2017 BP guideline over-extended the reach of hypertension diagnosis and pharmacologic treatment.25–28 The lower BP thresholds used to define hypertension indeed lead to more diagnoses.29 However, because the recommendation for antihypertensive drug treatment in patients with pre-treatment BP 130–139/80–89 mmHg was limited to those at high CVD risk, treatment under the 2017 guideline would lead to more health gains while only extending treatment to 5.4% more adults with hypertension compared to JNC7.2 Based on the 2017 guideline recommendations, adults with stage one hypertension for whom initiation of pharmacological antihypertensive treatment is recommended have a six-fold higher rate of CVD events compared with their counterparts who have also have stage one hypertension but are too low risk to qualify for antihypertensive drug therapy.30 The range of treatment efficiency we estimated among 2017 guideline treatment groups can be used by hypertension control programs to prioritize highest treatment efficiency groups for resource allocation and early guideline implementation. We demonstrated in prior publications that treating hypertension is either cost-effective or cost-saving in most adults and, despite higher initial treatment costs and the potential for SAEs, more intensive SBP treatment is cost-effective over a lifetime compared to standard treatment in high CVD risk individuals.31, 32
The results of the current analysis are consistent with a prior modeling study by Bundy et al. that, projected that achieving and maintaining 2017 ACC/AHA guideline-recommended BP goals would prevent twice as many CVD events than achieving and maintaining JNC8 PM goals among US adults age ≥40 years.33 Similar to that analysis, we used a network meta-analysis of 42 randomized BP lowering trials including 144,220 participants as the source of CVD risk reductions associated with achieving recommended versus current systolic BP levels.3 However, in their analysis, Bundy et al. based treatment group sizes on NHANES 2013–2016 and CVD event rates on older cohort studies (i.e., the Atherosclerosis Risk in Communities [ARIC], Framingham Offspring Study, Cardiovascular Health Study [CHS], and Multi-Ethnic Study of Atherosclerosis [MESA]; enrollment dates ranging from 1985–2004) and calibrated the CVD event rates to match rates reported in the AHA 2015 Heart Disease and Stroke Statistics. These input decisions resulted in a uniformly higher baseline event rates than were entered into our models. We choose to use the REGARDS study as our source for CVD events rates as REGARDS represents one of the largest, most contemporary population-based samples of US adults with rigorously adjudicated CVD events with ten years of follow-up. Accurate CVD incidence rates are difficult to determine at a national population level.
Even though the population health benefits could be substantial, and treatment efficiency would be acceptable, the expected benefits of achieving the 2017 ACC/AHA guideline BP goals must be weighed against the potential harms of pharmacological treatment. We estimated that achieving and maintaining the 2017 guideline goals could cause the same number of SAEs as the number of CVD events prevented (about three million over ten years). However, CVD events and SAEs are generally not equivalent in severity and should not be directly compared. A proportion of CVD events are fatal or non-fatal but severely disabling, whereas the overwhelming majority of SAEs (e.g., syncope, hypotension, and electrolyte abnormalities) are non-fatal and the acute kidney injury follow-up experience in SPRINT suggest many SAEs are mild transient events from which participants make a complete recovery within twelve months.34 Evidence regarding the harms of antihypertensive treatment, especially intensive therapy, is more limited than evidence of benefit. Until recently, reporting on harms in many BP-lowering trials has not been rigorous or standardized. The treatment-related SAE rates used in our analysis were from contemporary trials in high CVD risk patients that are among the few that carefully recorded and ascertained SAEs.10, 11 The favorable balance between benefits and harms observed in the Hypertension in the Very Elderly Trial (HYVET), Systolic Hypertension in the Elderly Program (SHEP), and the SPRINT elderly participants support the 2017 ACC/AHA guideline recommendation of the same BP treatment goals in most ambulatory older persons and all other adults.35–37 However, the 2017 ACC/AHA guideline recommended that healthcare providers exercise caution when initiating or intensifying treatment in older adults. Until SAE risk prediction tools are implemented in clinical practice, “clinical judgment” will be the recommended approach to assess the potential for antihypertensive medication SAE risk in individual patients.
Achieving and maintaining the hypertension treatment and BP control goals recommended in the 2017 ACC/AHA BP guideline and simulated in our analysis will be challenging, but systematic, multi-component interventions can improve hypertension control dramatically.38 Integrated health systems such as Kaiser Permanente of Northern California and the Veterans Health Administration have achieved control rates of >80–90% for systolic/diastolic BP targets goals of <140/90 mmHg.39, 40 A recent meta-analysis of 121 randomized trials of practice-based interventions to improve BP control found that multilevel, multi-component strategies involving team-based care with antihypertensive medication titration by nurses or pharmacists resulted in a large systolic BP reduction (mean −7.1 mmHg over a median of 6 months).38 Adoption of the 2017 guideline treatment goals should be pursued on a platform of high quality in- and out-of-office BP measurement.1
This report draws on several major strengths. Effectiveness estimates for BP lowering were obtained from a recent network meta-analysis of 42 BP lowering trials involving 144,200 participants.3 Findings in the network meta-analysis were consistent whether or not SPRINT results were included.3 Because our goal was to generate estimates that are generalizable to the US adult population, we used NHANES, a nationally representative survey of the US adult population, and REGARDS, the most contemporary population-based sample of US adults with rigorously adjudicated CVD events. The current analysis also has important limitations. Although the REGARDS study enrolled US adults from the 48 contiguous US states, blacks and residents from the Southeastern US are over-represented by design. Therefore, the CVD event rates may be higher in the REGARDS study compared with the general US population. However, we reweighted the REGARDS population to match age, sex, and race distribution in NHANES. Another limitation is that we did not account for future changes in blood pressure, antihypertensive medication use, or other covariates. However, our analysis used the absolute 10-year CVD event rates from REGARDS and hazard ratios derived from intention-to-treat analyses of clinical trials, both of which incorporate time-varying changes in antihypertensive medications, blood pressure levels, and other characteristics over time. Therefore, had we adjusted for these time-varying changes in our analysis, we may have overestimated the number of CVD events prevented. The current analysis was limited to adults ≥ 45 years of age. We assumed no heterogeneity of treatment effect in terms of relative risk reduction across subgroups. About 51% of US adults with hypertension currently have controlled BP using the 140/90 mmHg goal (70–90% control in treated patients), so 100% implementation of the 2017 ACC/AHA guideline is unlikely in the short term and represents a “best-case” scenario.39–42 Both NHANES and REGARDS based their BP estimates on averages at a single visit in contrast to the ≥2 measurements over ≥2 occasions recommended in the 2017 ACC/AHA and JNC7 guidelines. REGARDS is not directly representative of the US adult population and has the potential to over- or under-estimate CVD risk. We assumed that higher risk of specific treatment-related SAEs in the intensive, compared with standard blood pressure treatment arm in SPRINT and ACCORD-BP were valid representations of intensive antihypertensive drug treatment SAE risk in community-dwelling patients. However, this likely represents a conservative estimate as there was no overall difference in SAEs between the intensive and standard treatment arms in SPRINT and, due to the unblinded nature of the study, the potential for bias in ascertainment of SAEs is a serious concern (i.e., favoring more SAE reporting in the intensive arm).
In conclusion, the results from this analysis suggest that achieving and maintaining the 2017 ACC/AHA guideline-recommended BP goals could prevent about three million CVD events over ten years when compared to current BP levels, but implementing the 2017 guideline could also lead to about three million more treatment-related SAEs.
Supplementary Material
CLINICAL PERSPECTIVES.
- What is new? (no more than 100 words)
- The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure (BP) guideline recommended lower BP thresholds for antihypertensive medication initiation and intensification for most patients with hypertension.
- We projected the potential population health impact of implementing the 2017 guideline among US adults age ≥45 years with hypertension compared with current BP levels and with prior guidelines.
- Achieving and maintaining 2017 guideline goals over ten years could prevent 3.0 million cardiovascular disease events—a greater number of events prevented compared with prior guidelines—but could also lead to 3.3 million more treatment-related serious adverse events.
- What are the clinical implications? (no more than 100 words)
- Achieving and maintaining 2017 ACC/AHA BP goals could prevent a greater number of CVD events than by achieving prior U.S. guideline goals but could also lead to more serious adverse events.
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of NHANES and the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
SOURCES OF FUNDING
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional support was provided by grants R01 HL080477, K24 HL125704, and K24 HL111154 from the National Heart, Lung, and Blood Institute, 15SFRN2390002 from the American Heart Association, and P20GM109036 (Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases) from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
Dr. Bress is supported by K01HL133468 and R01HL139837 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Dr. Moran is supported by R01 HL 130500–01A1 and R01HL139837 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Dr. Bibbins-Domingo is supported by K24DK103992. Dr. Bellows is supported by K01HL140170 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Drs. Bress, Colantonio, Muntner, and Moran had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
DISCLOSURES
JNB receives support from American Heart Association Strategically Focused Research Network grant 15SFRN2390002. AB has received research support to his institution from Novartis not related to the current project. EBL, MMS and PM have received research support from Amgen, Inc. unrelated to this paper. EBL also has served on advisory boards for Amgen, Inc. and has been a consultant for a research project funded by Novartis unrelated to this paper. PM also has received research honoraria from Amgen, Inc. unrelated to this paper.
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr. and Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK and He J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A and Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G and Messerli FH. Optimal Systolic Blood Pressure Target After SPRINT: Insights from a Network Meta-Analysis of Randomized Trials. Am J Med. 2017;130:707–719 e708. [DOI] [PubMed] [Google Scholar]
- 6.Banon D, Filion KB, Budlovsky T, Franck C and Eisenberg MJ. The usefulness of ranolazine for the treatment of refractory chronic stable angina pectoris as determined from a systematic review of randomized controlled trials. Am J Cardiol. 2014;113:1075–1082. [DOI] [PubMed] [Google Scholar]
- 7.Sussman J, Vijan S and Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S and Wright JT Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS and Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 10.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC and Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman EZ, Howard G, Cushman M, Kissela B, Kleindorfer D, Le A, Judd S, McClure LA and Howard VJ. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2012;59:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prineas RJ, Crow RS and Blackburn HW. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Boston, Mass.: J. Wright; 1982. [Google Scholar]
- 14.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H, Epidemiology AHACo, Prevention, Committee AHAS, World Heart Federation Council on E, Prevention, European Society of Cardiology Working Group on E, Prevention, Centers for Disease C, Prevention, National Heart L and Blood I. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, White HD, Joint ESCAAHAWHFTFftRoMI, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D and Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 16.Stroke−−1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94:496–509. [Google Scholar]
- 18.LaVange LM, Steams SC, Lafata JE, Koch GG and Shah BV. Innovative strategies using SUDAAN for analysis of health surveys with complex samples. Statistical Methods in Medical Research. 1996;5:311–329. [DOI] [PubMed] [Google Scholar]
- 19.Chew DP, Huynh LT, Liew D, Astley C, Soman A and Brieger D. Potential survival gains in the treatment of myocardial infarction. Heart. 2009;95:1844–1850. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Hernandez AF, Solomon SD and Yancy CW. Potential Mortality Reduction With Optimal Implementation of Angiotensin Receptor Neprilysin Inhibitor Therapy in Heart Failure. JAMA Cardiol. 2016;1:714–717. [DOI] [PubMed] [Google Scholar]
- 21.Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, Bansal VK, Cao G, Yee J, Moran AE, Durazo-Arvizu R, Muntner P and Cooper RS. Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey). Circulation. 2017;135:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law M, Morris J and Wald N. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. Bmj. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Archives of Internal Medicine. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 24.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 25.Wilt TJ, Kansagara D and Qaseem A. Hypertension Limbo: Balancing Benefits, Harms, and Patient Preferences Before We Lower the Bar on Blood Pressure. Ann Intern Med. 2018;168:369. [DOI] [PubMed] [Google Scholar]
- 26.Bakris G and Sorrentino M. Redefining Hypertension - Assessing the New Blood-Pressure Guidelines. N Engl J Med. 2018;378:497–499. [DOI] [PubMed] [Google Scholar]
- 27.Messerli FH and Bangalore S. Lowering the Thresholds of Diseases: Is Anyone Still Healthy? J Am Coll Cardiol. 2018;71:119–121. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM. Blood pressure guidelines as starting point in clinical decisions. BMJ. 2018;360. [DOI] [PubMed] [Google Scholar]
- 29.Pickering TG. Now we are sick: labeling and hypertension. The Journal of Clinical Hypertension. 2006;8:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colantonio LD, Booth JN 3rd, Bress AP, Whelton PK, Shimbo D, Levitan EB, Howard G, Safford MM and Muntner P. 2017 ACC/AHA Blood Pressure Treatment Guideline Recommendations and Cardiovascular Risk. J Am Coll Cardiol. 2018;72:1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, Berlowitz DR, Conroy MB, Fine L, Oparil S, Morisky DE, Kazis LE, Ruiz-Negron N, Powell J, Tamariz L, Whittle J, Wright JT Jr., Supiano MA, Cheung AK, Weintraub WS, Moran AE and Group SR. Cost-Effectiveness of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2017;377:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins-Domingo K, Coxson PG and Goldman L. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bundy JD, Mills KT, Chen J, Li C, Greenland P and He J. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. 2018;3:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, Umanath K, Rahbari-Oskoui F, Porter AC and Pisoni R. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). American Journal of Kidney Diseases. 2018;71:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 36.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ and Group HS. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 37.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr., Pajewski NM and Group SR. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, Cooper LA and He J. Comparative Effectiveness of Implementation Strategies for Blood Pressure Control in Hypertensive Patients: A Systematic Review and Meta-analysis. Ann Intern Med. 2018;168:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe MG, Lee GA, Young JD, Sidney S and Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe MG and Young JD. The Kaiser Permanente Northern California Story: Improving Hypertension Control From 44% to 90% in 13 Years (2000 to 2013). The Journal of Clinical Hypertension. 2016;18:260–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y and Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon SS, Carroll MD and Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 43.Odden MC, Peralta CA, Haan MN and Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothman MD, Leo-Summers L and Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED and Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. [DOI] [PubMed] [Google Scholar]
- 46.Cooper R, Kuh D, Hardy R, Mortality Review G, Falcon and Teams HAS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.