Figure 1.
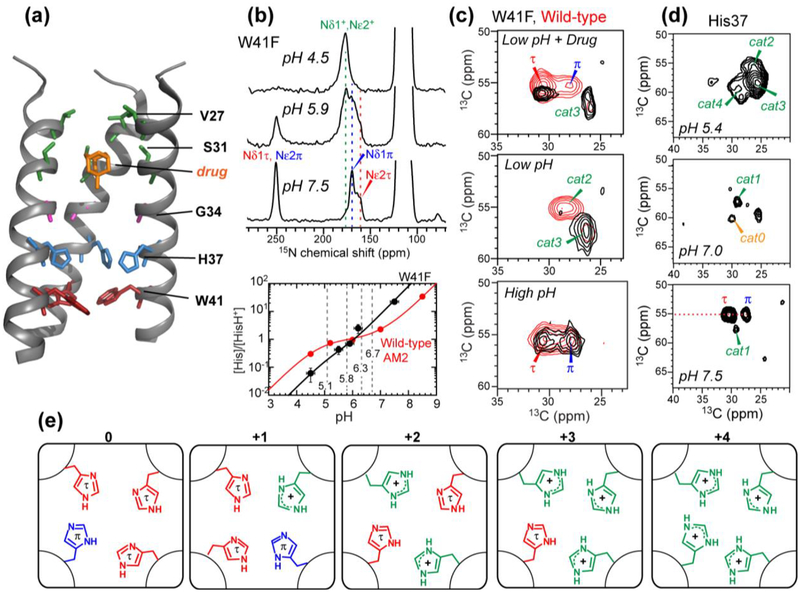
Structural studies of the influenza M2 proton channel by SSNMR. (a) The TM domain structure with bound drug (PDB: 2KQT), solved in DMPC bilayers. Important functional residues are indicated. (b) Determination of His37 pKa’s from pH-dependent His37 sidechain 15N chemical shifts. The W41F mutant data is shown as an example. Titration curves yield the His37 pKa’s, which differ for the W41F mutant and wild-type channels. (c) Histidine Cα and Cβ chemical shifts from 2D 13C-13C correlation spectra. A W41F mutation (black spectra) changes the His37 chemical shifts compared to the wild-type (red spectra), indicating that His37 can be protonated from the C-terminus in the mutant. (d) 2D 13C-13C correlation spectra of cytoplasmic-containing M2 as a function of pH. Seven states of His37 are observed. (e) Proposed His37 tetrads with different charge states, explaining the resolved seven sets of histidine chemical shifts.