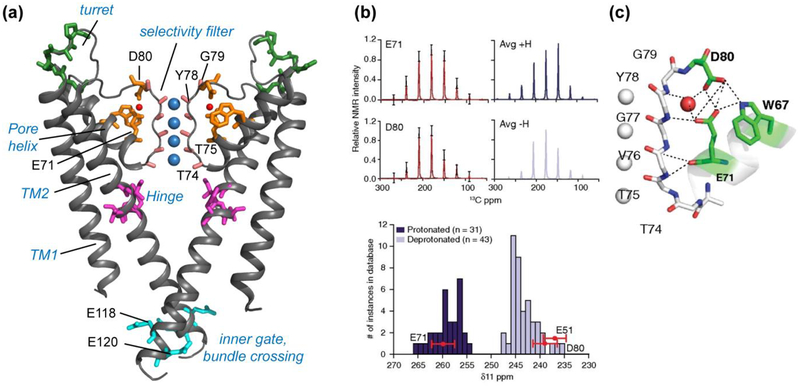
Figure 2.
KcsA structure investigated using SSNMR. (a) Key domains and functional residues in KcsA that have been studied (PDB: 1K4C). (b) Measured sidechain carboxyl chemical shift anisotropies (CSA) (black) of E71 and D80, with the simulated CSA patterns overlaid in red (5). The simulated spectra of average protonated (dark blue) and deprotonated (light blue) carboxyl CSA tensors are shown on the right, based on a database of known crystal structures. Compared to this database, the δ11 component of E71 matches with protonated (dark blue) carboxyls, while E51 and D80 δ11 components match with deprotonated (light blue) carboxyls, indicating an elevated pKa for E71. (c) The hydrogen-bonding network connecting the selectivity filter and the pore helix in the high K+ conductive state (PDB: 1K4C). Panels (b) and (c) reproduced with permission from Reference (5).