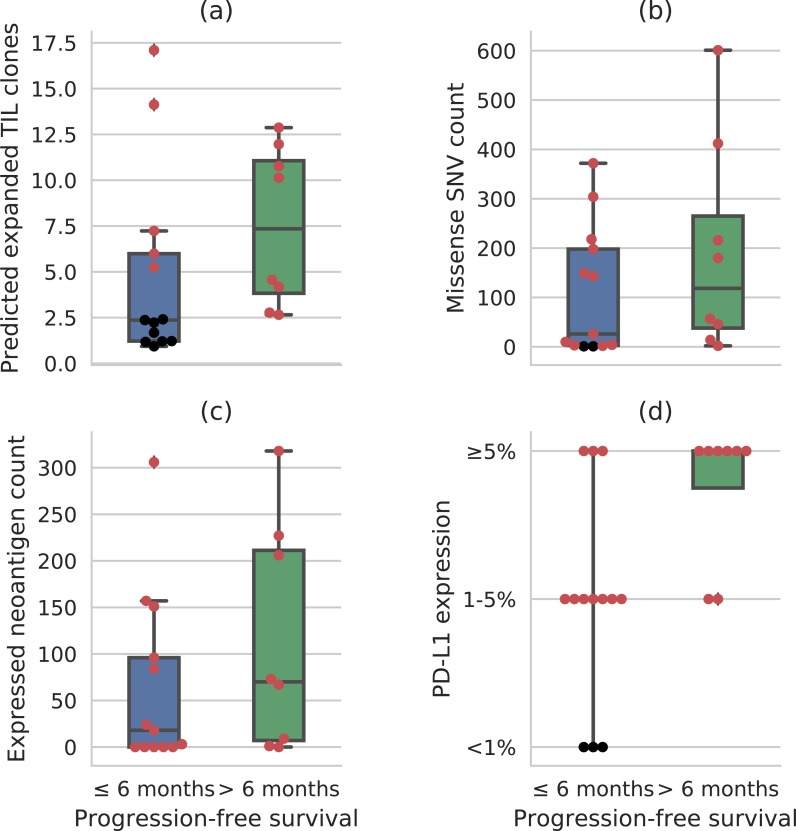
Fig 3.
Distributions of biomarker values in patients with and without durable clinical benefit (DCB, defined as ≥ 6 months of progression-free survival): (A) predicted number of expanded TIL clones; (B) missense SNV count; (C) expressed neoantigen count; and, (D) percentage of tumor infiltrating immune cells found to be PD-L1-positive. When each biomarker alone is used for triage, the patients highlighted in red must be treated to ensure all DCB patients are treated.