Abstract
Vector-borne pathogens are responsible for serious emerging diseases and have been widely described in wildlife. Ehrlichia chaffeensis causes the zoonotic “monocytic ehrlichiosis” in humans, is transmitted by the tick Amblyomma americanum and its reservoir host is the white-tailed deer (Odocoileus virginianus) in North America. Little is known about the native reservoir and the tick vectors involved in the transmission cycle in South America. We report here the detection of E. chaffeensis in a study on marsh deer (Blastocerus dichotomus) mortality in Argentina, in different time periods between 2007 and 2016. Four deer, from two distinct populations, were positive for E. chaffeensis through molecular methods. Additionally, the variable-length PCR target (VLPT) region of positive samples was genotyped. Our results provide the first evidence of E. chaffeensis in autochthonous Cervidae from Argentina, contributing to uncover the distribution of this tick-borne infection in South America.
Keywords: Ehrlichia chaffeensis, Ticks, Marsh deer, Blastocerus dichotomus
Graphical abstract
Highlights
-
•
First evidence of E. chaffeensis in autochthonous cervidae from Argentina.
-
•
Two geographically separated marsh deer populations were positive for E. chaffeensis.
-
•
Genotype characterization over time reveals persistent circulation of E. chaffeensis.
1. Introduction
Within the emerging tick-borne infections, ehrlichioses are potentially zoonotic diseases that stand out because of the multiple vertebrate hosts and hard tick vectors that are involved in their transmission cycle. Like other tick-borne diseases, ehrlichioses are strongly influenced by climate change because it may alter the density, activity patterns and geographic distribution of arthropod and hosts involved (Randolph, 2010). The genus Ehrlichia comprises Gram-negative intracellular bacteria from the Anaplasmataceae family, which are transmitted by hard ticks (Dumler et al., 2001). This genus currently includes recognized species (E. canis, E. chaffeensis, E. ewingii, E. muris, E. ovis, E. ruminantium) and potentially new species under characterization (Tate et al., 2013; Pritt et al., 2017; Yang et al., 2017).
Ehrlichia chaffeensis is responsible for the symptomatic monocytic ehrlichiosis in humans (Paddock and Childs, 2003). In the United States Amblyomma americanum ticks are the transmitting vectors of E. chaffeensis and the white-tailed deer (Odocoileus virginianus) is the main reservoir host (Ewing et al., 1995), while humans and dogs are incidental hosts. Bovines are susceptible to experimental infection with E. chaffeensis (Delos Santos et al., 2008).
In Argentina Tomassone et al. (2008) detected E. chaffeensis in three developmental stages of Amblyomma parvum tick through molecular methods and Ripoll et al. (1999) reported serological evidence of E. chaffeensis in humans in the north-west region of the country. The lack of simultaneous evidence of E. chaffeensis in mammalian host and arthropods has prevented the recognition of the local species capable of maintaining and transmitting the pathogen respectively. In this study, we analyzed the occurrence of E. chaffeensis in marsh deer (Blastocerus dichotomus) that inhabit the Paraná River Delta and Ibera Wetlands in Argentina.
2. Materials and methods
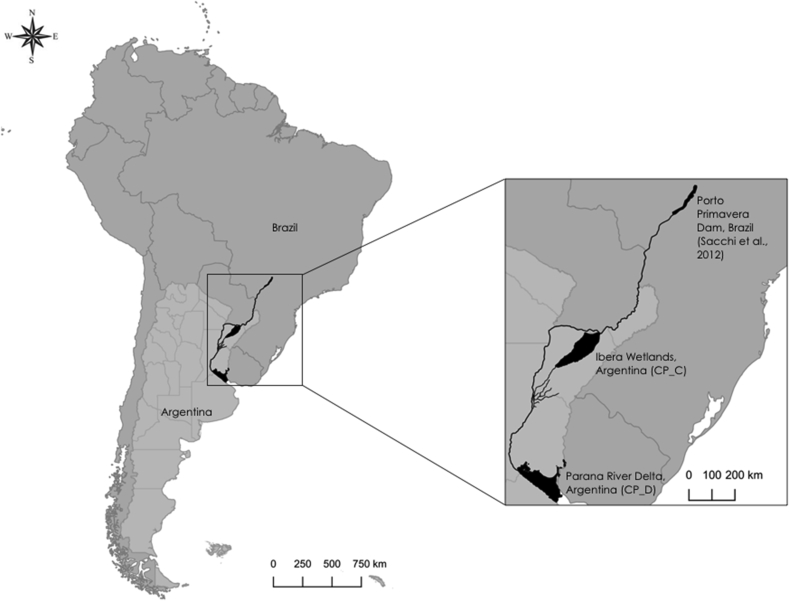
Samples were collected during mortality events of marsh deer in 2007, 2015 and 2016 in two different populations located along the alluvial plain of the Paraná River, in Argentina: Paraná River Delta and Ibera Wetlands. The Paraná River Delta region, located between 60° 39′ W, 32° 60′ S and 58° 30′ W, 34° 30′ S, is a complex floodplain covered by herbaceous vegetation on frequently flooded area, shrub savannas and forests highly disturbed by afforestation, cattle rearing and fishing (Malvárez, 1999, Iriondo, 2004) (Fig. 1, Parana River Delta). The Ibera Wetlands, which lay on the paleolithic-river beds of the Parana River between 56° 25′ W, 27° 30′ S and 58° W 29° S in Corrientes province, is about 800 km North to Parana River Delta and rather pristine. (Fig. 1, Ibera Wetlands). It is a temporarily and permanently waterlogged area, combined with forests, scrublands, grasslands, pastures, lakes, wetlands and peat lands. Remarkably, both areas have no hydrographic connection between each other.
Fig. 1.
Map of the two marsh deer populations (Paraná River Delta and Ibera Wetlands) located along the alluvial plain of the Paraná River (Argentina).
Complete necropsies of succumbed marsh deer were performed. All deer showed cachexia and signs of anemia and were clearly weakened (Orozco et al., 2013). Full blood samples from the heart of recently succumbed animals (within the hour) were collected and stored with EDTA: a 2-ml aliquot was refrigerated at 4 °C and 1 ml was frozen at −20 °C. Right afterwards, the succumbed deer was examined for ticks, which were removed and stored in 70% ethanol. Tick examination was conducted by visual inspection on predilection sites (face, ears, brisket, withers, knees, perineum region and tail). The tick burden was roughly estimated only in recently dead marsh deer. Biosafety procedures for animal management were performed according to protocols approved by the Argentinean CICUAL (Institutional Committee for the Care and Use of Experimental Animals; Protocol N° 2014-40, issued by the Facultad de Ciencias Veterinarias, Universidad de Buenos Aires). Wildlife permits (including transit permits for biological samples) were obtained from the provincial government through “Natural Resources Agency of Corrientes” (Disposition N° 845, Proceeding N° 193-15-09-410/2014) and the General Directorate for Natural Resources of Entre Rios (Authorization N° 007/16/Resolution 1721/14 D.G.R., Proceeding N°, 1845661).
The ticks were taxonomically identified (Guglielmone and Viñabal, 1994; Nava et al., 2014) under a stereoscopic magnifier (10X-40X) before processing. DNA was extracted by the phenol/chloroform method followed by a standard ethanol precipitation whether from crushed ticks using liquid nitrogen or 400 μl of whole blood (Halos et al., 2004). DNA quality and concentration were determined using a micro-volume spectrophotometer (NanoDrop ND-1000. ThermoFisher Scientific).
Detection of E. chaffeensis DNA from the blood samples and ticks was conducted with genus- and species-specific primers. Initially, all samples were screened using a PCR protocol targeting the 16S rRNA gene common to Ehrlichia spp. and Anaplasma spp. (Bekker et al., 2002). Subsequently, positive samples were also tested employing a specific 16S rRNA E. chaffeensis Forward primer (Anderson et al., 1992) together with the Reverse primer employed previously (Bekker et al., 2002). Lastly, the variable-length PCR target (VLPT) region of positive samples was genotyped (Sumner et al., 1999).
The PCRs were performed in a 50 μl reaction mixture (0.4 μmol of each primer, 0.2 mM of each deoxyribonucleotide triphosphate, 1.25 U of GoTaq DNA polymerase - Promega Madison, Wi. USA, 10 μl of 5x PCR buffer and purified water for 50 μl of final volume) using 200 ng of genomic DNA (both for blood and tick samples). Amplification was carried out in a thermocycler (Bio-Rad MyCycler Thermal Cycler) under specific cycling conditions (Anderson et al., 1992; Sumner et al., 1999; Bekker et al., 2002). For each amplification reaction, positive (DNA from cultured E. chaffeensis Arkansas strain) and negative (pure water) controls were included. An aliquot of 5 μl of each amplified product was analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide. A molecular size marker (1 Kb Plus DNA Ladder, Invitrogen) was used to determine PCR product size.
Both strands from 16S rRNA Ehrlichia/Anaplasma and VLPT fragments were sequenced with a Big Dye Terminator v3.1 kit from Applied Biosystems and analyzed on an ABI 3130XL genetic analyzer from the same supplier (Genomic Unit, Consorcio Argentino de Tecnología Genómica (CATG), Instituto de Biotecnología, CICVyA, INTA).
Raw files from target regions were processed using the Vector NTI Advanced 10 program (Invitrogen). For each fragment, forward and reverse chromatograms (ab1 files) were used for assembling and the outcome contig (FASTA file) was used for further sequence analysis.
3. Results
In this study, we assessed the E. chaffeensis presence in blood samples from B. dichotomus (n = 38) as well as in ticks Amblyomma triste (n = 7) and Rhipicephalus microplus (n = 173) from 7 succumbed deer (Table 1).
Table 1.
Number of sampled deer and tick species collected in Ibera Wetlands and Parana River Delta deer populations. Numbers between brackets refer to the number of ticks found.
| Samples obtained | Number of marsh deer |
Total | |
|---|---|---|---|
| Ibera Wetlands CP_C | Parana River Delta CP_D | ||
| Deer blood | 17 | 21 | 38 |
| Rhipicephalus microplus ticks | 5 (n = 173) | 0 | 5 (n = 173) |
| Amblyomma triste ticks | 1 (n = 2) | 2 (n = 5) | 3 (n = 7) |
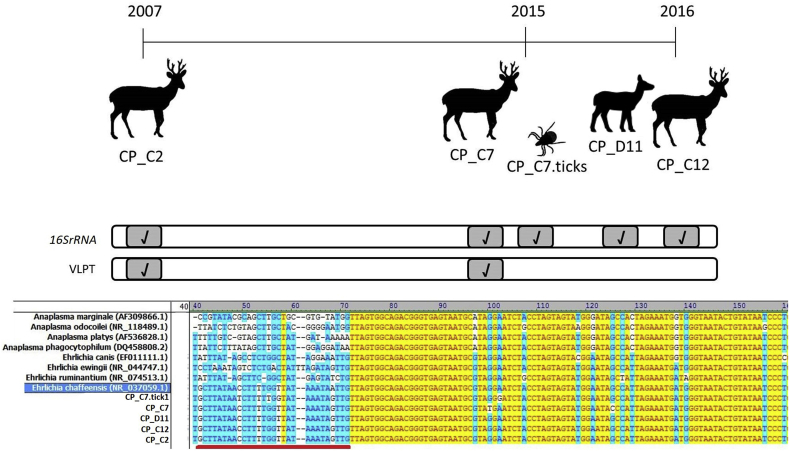
From the 38 tested deer blood samples, 4 were positive to the generic 16S rRNA (KY644143-KY644146) and the E. chaffeensis-specific PCR reaction. Five adult R. microplus from a positive deer (CP_C7) tested positive for E.chaffensis 16SrRNA. The remaining 168 R. microplus and A. triste (n = 7) tested negative for generic 16SrRNA (Fig. 2). Although the PCR reaction rendered the expected size for the 16SrRNA target region, only one of the positive tick samples contained enough DNA for sequencing (KY644147). The obtained 16S rRNA 410 bp fragment, which hold the hypervariable V1 region, showed high identity values with E. chaffeensis strains deposited in GenBank (Table 2). The V1 region alignment strengthened species identification (Fig. 2, bottom).
Figure 2.
a_Summary of the E. chaffeensis molecular amplification of the 16SrRNA and the VLPT genes in marsh deer and tick samples at different time points. b_ Alignment of a fragment of the 16SrRNA nucleotide sequences from reference Anaplasma and Ehrlichia strains and those amplified from deer (C2, C7, D11 and C12) and a tick (tick1_C7) in the present study. The red line marks the hypervariable V1 region.
Table 2.
Results of the global nucleotide alignment of deer sample sequences in the present study.
To further characterize the positives samples, we then amplified the variable-length PCR target (VLPT). This assay yielded an amplicon for two samples (blood from CP_C2 and CP_C7 from Ibera) (Fig. 2). We subsequently compared the protein sequences retrieved for the VLPT gene (KY652924 - KY652925) against two previously reported polymorphic regions (EU826517 and EU826518) amplified from ticks collected in Argentina (Tomassone et al., 2008) and the E. chaffeensis strain Arkansas (WP011452439.1). The new target sequences revealed a novel genotype that was identical for both samples but different from the formerly reported sequences (Table 3).
Table 3.
Aminoacid sequence of VLPT tandem repeats and profiles for previously reported E. chaffeensis in Argentina (EU826517 and EU826518), CP_2 and CP_7 deer samples and the E. chaffeensis positive control Arkansas strain (Reference).
| Repeat sequence | Sample Name |
||||
|---|---|---|---|---|---|
| EU826517 | EU826518 | CP_C2 | CP_C7 | Arkansas strain | |
| SDSHESSHTVPNLSEEVVQLESELQQS | ✓ | ✓ | ✓ | ✓ | |
| SDFDLQGIFSVELFDPFKDAVQLGNDLQHS | ✓ | ✓ | ✓ | ✓ | |
| SNSDYRSSPVELPGPSKEEVQLESAVQP | ✓ | ✓ | ✓ | ✓ | |
| SDSGLHGPSHLELPSLAEEVMQLEDDLQQP | ✓ | ✓ | ✓ | ||
| SDFDLQGIFSVELFDPFKEAVQLGNDLQQP | ✓ | ✓ | |||
| SDFDLQGIFSVELPSPSKEEVQLENDTKNVVY | ✓ | ✓ | |||
| SDSGLHGPSHLELPSLAEEVMQLEDDLQQS | ✓ | ||||
| SFGLHRSSSVELPSPSKEEVQLENDTKNVVY | ✓ | ✓ | |||
| SDFDLQGIFSVELFDPFKEAVQLGNDLQQS | ✓ | ||||
| SDSHEPSHLELPSLSEEVIQLESDLQQSSN | ✓ | ||||
| SDLHGSFSVELFDPFKEAVQLGNDLQQSSD | ✓ | ||||
| SDLHGSFSVELFDPSKEEVQLESDLQQSSN | ✓ | ||||
| SDLHESSFVELPGPSKEEVQFEDDAKNVVY | ✓ | ||||
The E. chaffensis positive blood samples were collected in 2007, 2015 and 2016 from three adult deer (CP_C2, CP_C7 and CP_C12) and one newborn deer (CP-11). The adults deer died during a mortality event in Ibera Wetlands and had high R. microplus tick infestation (more than 50 ticks grouped in one or more predilection sites), with cutaneous lesions and exudation of tissue fluid, especially on external ears and face (Fig. 3). The deer CP_D11 was a newborn deer dead by starvation after illegal hunters killed his mother during the floods of 2016 in the Paraná River Delta. No ticks were detected on CP_D11 (Fig. 2).
Fig. 3.
Dead marsh deer with high tick burden.
4. Discussion
In our study, E. chaffeensis DNA was detected in blood samples from two geographically distinct marsh deer population during mortality events at different time points in 2007, 2015 and 2016, providing the first evidence of E. chaffeensis presence in a mammalian host in Argentina. E. chaffeensis has also been reported in some Brazilian Parana river marsh deer population (Fig. 1) (Machado et al., 2006; Sacchi et al., 2012), but we could not use the sequences for comparison (DQ345720.1 and JQ085940.1) as the authors targeted a different 16S rRNA fragment that does not contain the V1species specific region (Dumler et al., 2001).
Although in South America E. chaffeensis can be present in ticks from domestic and wild mammals (Tomassone et al., 2008), little is known about the native mammalian hosts and vectors involved in its transmission cycle. White-tailed deer are the most common host for the three mobile stages of A. americanum (Yabsley, 2010) in USA. The exposure to the agent appears to occur early in the life in this host species (Paddock and Yabsley, 2007) and ticks can become infected by feeding on animals with a persistent bacteremia without apparent clinical signs of disease (Davidson et al., 2001). In North America, E. chaffeensis has been also found in A. maculatum, Dermacentor variabilis and Ixodes pacificus among others (Paddock and Yabsley, 2007).
We found E. chaffeensis DNA in the main B. dichotomus populations in Argentina, where A. triste is endemic. Even though E.chaffeensis was apparently absent from the collected A. triste (n = 7), we expect that the agent will be detected if a large sample is analyzed. Ticks feeding on infected deer could be acquiring the organism in a blood meal but not necessarily transmitting or maintaining the infection. Whereas whole full engorged R. microplus from infected marsh deer were positive for E. chaffeensis DNA, further studies should be performed so as to appraise for its vector competence.
From an epidemiological point of view, correlation between samples and geographic origin together with variations in gene sequences under immunological selection are used as markers for tracking infection cycles (Sumner et al., 1999). For revealing the intraspecific variation of circulating isolates, we sequenced the VLPT gene fragment, a size variation gene resulting from loss or gain of long of direct repeats within the protein coding sequences (Table 3). In this regard, we found that the Ibera isolates were the same genotype over time, though different from the genotypes previously detected in A. parvum ticks from Argentina (Tomassone et al., 2008) (Table 3). Up to now, the significance of the variable number of repeats in VLPT genes among isolates remains unclear (Cheng et al., 2003), but in the present context we could consider the genotypic relatedness over time as confident evidence of persistent circulation of E. chaffeensis.
As a whole, the results shown in the present report provide relevant data to highlight awareness of ehrlichiosis in Argentina. More evidence from comprehensive studies over time and space that include sampling of human, domestic animals, wildlife and vectors would be greatly beneficial to understand the spread and abundance of the pathogen in Argentina and draw definite conclusions.
5. Conclusions
The findings of E. chaffeensis in B. dichotomus, during independent mortality events over time and in two geographic areas, suggest that E. chaffeensis is circulating in marsh deer populations from Argentina. Previous reports of E. chaffeensis in A. parvum ticks, serological evidence in humans and our present findings provide valuable information to understand E. chaffeensis epidemiology in Argentina. Forthcoming studies should comprise larger sampling, including a wide range of hosts and vectors in order to determine the extent of the E. chaffeensis in the region.
Acknowledgements
We are grateful to Gabriel Ruiz Díaz, Mariela Pilar Morales, Pascual Perez and Pablo Rodriguez for field assistance. We also thank to Benjamin Cuomo, Gabriel Tato (Municipality of San Fernando), Claudio Ledesma, Santiago D'Alessio and Vicente Fraga for providing support during mortality episodes of marsh deer. We thank William Nicholson for providing us with positive controls. We are deeply grateful to Pablo Rodriguez, Sebastián Raviculé and Gisella Müller for the photography (Graphical abstract and Fig. 3). This work was financed by grants from the Argentine Ministry of Science and Technology (PIP 11220120100108CO, CONICET and PICT 2015–2001) and INTA PNBIO (1131043 and 1131032). MMO and MDF are members of CONICET Researcher’s Career.
References
- Anderson B.E., Sumner J.W., Dawson J.E., Tzianabos T., Greene C.R., Olson J.G., Fishbein D.B., Olsen-Rasmussen M., Holloway B.P., George E.H. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 1992;30(4):775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker C.P., de Vos S., Taoufik A., Sparagano O.A., Jongejan F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002;89(2–3):223–238. doi: 10.1016/s0378-1135(02)00179-7. [DOI] [PubMed] [Google Scholar]
- Cheng C., Paddock C.D., Reddy Ganta R. Molecular heterogeneity of Ehrlichia chaffeensis isolates determined by sequence analysis of the 28-kilodalton outer membrane protein genes and other regions of the genome. Infect. Immun. 2003;71(1):187–195. doi: 10.1128/IAI.71.1.187-195.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W.R., Lockhart J.M., Stallknecht D.E., Howerth E.W., Dawson J.E., Rechav Y. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 2001;37(3):538–546. doi: 10.7589/0090-3558-37.3.538. [DOI] [PubMed] [Google Scholar]
- Delos Santos J.R., Oglesbee M., Rikihisa Y., Stich R.W. Pathologic evidence of ehrlichiosis in calves inoculated with Ehrlichia chaffeensis. Ann NYAcad Sci. 2008;1149:103–106. doi: 10.1196/annals.1428.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler J.S., Barbet A.F., Bekker C.P.J., Dasch G.A., Palmer G.H., Ray S.C., Rikihisa Y., Rurangirwa F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001;51(Pt 6):2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Ewing S.A., Dawson J.E., Kocan A.A., Barker R.W., Warner C.K., Panciera R.J., Fox J.C., Kocan K.M., Blouin E.F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J. Med. Entomol. 1995;32(3):368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Guglielmone A.A., Viñabal A.E. Claves morfológicas dicotómicas e información ecológica para la identificación de garrapatas del género Amblyomma Koch, 1844 de la Argentina. Revista de Investigaciones Agropecuarias. 1994;25:39–67. [Google Scholar]
- Halos L., Jamal T., Vial L., Maillard R., Suau A., Le Menach A., Boulouis H.J., Vayssier-Taussat M. Determination of an efficient and reliable method for DNA extraction from ticks. Vet. Res. 2004;35(6):709–713. doi: 10.1051/vetres:2004038. [DOI] [PubMed] [Google Scholar]
- Iriondo M. The littoral complex at the Paraná mouth. Quat. Int. 2004;114:143–154. [Google Scholar]
- Machado R.Z., Duarte J.M., Dagnone A.S., Szabo M.P. Detection of Ehrlichia chaffeensis in Brazilian marsh deer (Blatocercus dichotomus) Vet. Parasitol. 2006;139(1–3):262–266. doi: 10.1016/j.vetpar.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Malvárez A.I. El Delta del Río Paraná como mosaico de humedales. IV. In: Malvárez A.I., editor. Tópicos sobre humedales subtropicales y templados de Sudamérica. Oficina Regional de Ciencia y Técnica para América Latina y el Caribe. MAB/UNESCO; Montevideo, Uruguay: 1999. pp. 35–54. [Google Scholar]
- Nava S., Beati L., Labruna M.B., Caceres A.G., Mangold A.J., Guglielmone A.A. Reassessment of the taxonomic status of Amblyomma cajennense with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae) Ticks Tick Borne Dis. 2014;5:252–276. doi: 10.1016/j.ttbdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Orozco M.M., Marull C., Jiménez Pérez I., Gürtler R. Winter mortality of marsh deer (Blastocerus dichotomus, Illiger 1815) in wetlands of northeastern Argentina. Mastozool. Neotrop. 2013;20(1):163–170. [Google Scholar]
- Paddock C.D., Childs J.E. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 2003;16(1):37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C.D., Yabsley M.J. Ecological havoc, the rise of white- tailed deer, and the emergence of Amblyomma americanum associated zoonoses in the United States. Curr. Top. Microbiol. Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- Pritt B.S., Allerdice M.E.J., Sloan L.M., Paddock C.D., Munderloh U.G., Rikihisa Y., Tajima T., Paskewitz S.M., Neitzel D.F., Hoang Johnson D.K., Schiffman E., Davis J.P., Goldsmith C.S., Nelson C.M., Karpathy S.E. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 2017;67(7):2121–2126. doi: 10.1099/ijsem.0.001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph S.E. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Vet. Parasitol. 2010;167(2–4):92–94. doi: 10.1016/j.vetpar.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Ripoll C.M., Remondegui C.E., Ordonez G., Arazamende R., Fusaro H., Hyman M.J., Paddock C.D., Zaki S.R., Olson J.G., Santos-Buch C.A. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am. J. Trop. Med. Hyg. 1999;61(2):350–354. doi: 10.4269/ajtmh.1999.61.350. [DOI] [PubMed] [Google Scholar]
- Sacchi A.B., Duarte J.M., André M.R., Machado R.Z. Prevalence and molecular characterization of Anaplasmataceae agents in free-ranging Brazilian marsh deer (Blastocerus dichotomus) Comp. Immunol. Microbiol. Infect. Dis. 2012;35(4):325–334. doi: 10.1016/j.cimid.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Sumner J.W., Childs J.E., Paddock C.D. Molecular cloning and characterization of the Ehrlichia chaffeensis Variable-Length PCR Target: an antigen-expressing gene that exhibits interstrain variation. J. Clin. Microbiol. 1999;37(5):1447–1453. doi: 10.1128/jcm.37.5.1447-1453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate C.M., Howerth E.W., Mead D.G., Dugan V.G., Luttrell M.P., Sahora A.I., Munderloh U.G., Davidson W.R., Yabsley M.J. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus) Ticks Tick Borne Dis. 2013;4(1–2):110–119. doi: 10.1016/j.ttbdis.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassone L., Nuñez P., Gürtler R.E., Ceballos L.A., Orozco M.M., Kitron U.D., Farber M. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks collected in northern Argentina. Emerg. Infect. Dis. 2008;14(12):1953–1955. doi: 10.3201/eid1412.080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley M.J. Natural History of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet. Parasitol. 2010;167(2–4):136–148. doi: 10.1016/j.vetpar.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu Z., Niu Q., Liu J., Han R., Guan G., Hassan M.A., Liu G., Luo J., Yin H. A novel zoonotic Anaplasma species is prevalent in small ruminants: potential public health implications. Parasites Vectors. 2017;10(1):264. doi: 10.1186/s13071-017-2182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]