Abstract
Purpose
The aim of this study was to evaluate the effects of various prophylactic treatments of titanium implants on bacterial biofilm formation, correlating surface modifications with the biofilms produced by Pseudomonas aeruginosa PAO1, Staphylococcus aureus, and bacteria isolated from saliva.
Methods
Pure titanium disks were treated with various prophylactic procedures, and atomic force microscopy (AFM) was used to determine the degree to which surface roughness was modified. To evaluate antibiofilm activity, we used P. aeruginosa PAO1, S. aureus, and saliva-isolated Streptococcus spp., Bacteroides fragilis, and Staphylococcus epidermidis.
Results
AFM showed that the surface roughness increased after using the air-polishing device and ultrasonic scaler, while a significant reduction was observed after using a curette or polishing with Detartrine ZTM (DZ) abrasive paste. In addition, we only observed a significant (P<0.01) reduction in biofilm formation on the DZ-treated implant surfaces.
Conclusion
In this study, both AFM and antibiofilm analyses indicated that using DZ abrasive paste could be considered as the prophylactic procedure of choice for managing peri-implant lesions and for therapy-resistant cases of periodontitis.
Keywords: Bacterial biofilm, Peri-implant diseases, Prophylactic procedures, Titanium
Graphical Abstract
INTRODUCTION
Implant-related infections are among the most important causes of dental implant failure. Although estimates of prevalence are quite variable due to inconsistent disease definitions [1], in a systematic review and meta-analysis, Derks and Tomasi [2] reported a weighted mean prevalence of peri-implant mucositis and peri-implantitis of 43% (95% confidence interval [CI], 32%–54%) and 22% (95% CI, 14%–30%), respectively.
As established at the sixth European Workshop on Periodontology (in 2008), peri-implant diseases are defined as “inflammatory reactions in the tissues surrounding the implants” and are frequently linked with the presence of a bacterial biofilm, leading in some cases to the loss of supporting bone, maxillary sinusitis, mandibular fracture, or the infection of other implants or natural teeth [3,4].
In general, the oral cavity, including the teeth, gingival sulcus, attached gingiva, tongue, cheek, lip, hard palate, and soft palate, with around 1,000 species [5], hosts the second most complex microbiota in the body after the colon. The presence and diversity of the oral microbiota are frequently associated with systemic diseases (e.g., diabetes, respiratory and cardiovascular disease, and osteoporosis) [6] and particularly with dental caries, periodontitis, and peri-implant diseases [7,8]. In a healthy patient who has received an implant, homeostasis exists between the peri-implant tissues and the microbial communities that have colonized the device. In the presence of inflammation, this delicate equilibrium can be disrupted in favor of proliferation and persistence of inflammation-triggering microbial communities, which may lead to the progression of peri-implantitis [9,10]. In such cases, several species of bacteria develop an adherent layer of plaque (referred to as a biofilm) on the implant surface, which protects the organized bacteria from both fluid shear stress and the action of systemic pharmacological therapies [11,12]. Biofilm formation involves a sequence of events starting with reversible adhesion of bacteria to a solid surface (tooth or dental implant), mainly due to electrostatic or Van der Waals forces established between bacteria and the material surface. The second stage consists of bacterial accumulation and irreversible attachment mediated by bacterial adhesion proteins and extracellular matrix formation. Finally, the biofilm matures with the detachment of some bacteria for new surface colonization. Implant surface properties (i.e., roughness, hydrophilicity, chemical composition, and even the sterilization method) can influence the first stage of biofilm formation [13]. It is well known that rough surfaces promote bacterial attachment and biofilm formation to a greater extent than smooth surfaces via the increased contact area between the material and bacterial cells and the protection of bacteria from shear forces [14,15]. Helpful therapeutic programs for the management of peri-implant diseases consist of non-surgical therapies, including prolonged antibiotic (which can last for years), antiseptic, and laser treatments, as well as regular oral hygiene of teeth and implants and periodic removal of microbial deposits from the implants. However, these treatments have shown non-predictable results and can ultimately lead to removal of the device, with a consequent increase in hospitalization time and cost, as well as a greater burden of patient compliance [16,17].
For this reason, the procedure of choice to avoid biofilm formation is the mechanical and/or ultrasonic debridement of titanium implant surfaces using air-abrasive devices, with or without the adjunctive use of chemical agents (i.e., irrigation with local disinfectants, and local or systemic antibiotic therapy) [18,19,20]. These treatments produce microscopically visible surface alterations dependent on the nature, particle size, and composition of the powder.
In the present study, we investigated the effects of 4 different prophylactic titanium implant treatments on bacterial biofilm formation, correlating surface texture modifications with the biofilms produced by Pseudomonas aeruginosa PAO1, Staphylococcus aureus, and bacteria isolated from saliva. The 2 commercially-available bacterial strains that were analyzed are opportunistic pathogenic bacteria frequently associated with peri-implant disease and implant failure [21,22].
P. aeruginosa PAO1 is one of the most important Gram-negative bacteria that cause biofilm-associated infections, particularly in immunocompromised persons, and is a well-established model organism for studying biofilm development. In addition, Canullo et al. [22] identified P. aeruginosa PAO1 in patients affected by peri-implant disease at the level of both the peri-implant sulcus and gingival sulcus of the adjacent teeth and the connection and abutment at the inner portion of each implant.
Furthermore, S. aureus is considered to be a major pathogen associated with medical device-related infections. Indeed, several studies have demonstrated that S. aureus has a high affinity for titanium surfaces and can be found in peri-implant lesions, as well as in therapy-resistant cases of periodontitis [23,24].
MATERIALS AND METHODS
Following several previous studies, we determined the sample size, assuming a statistically significant difference in optical density (OD; indicator of the adherent viable biomass) or log10 colony-forming unit (CFU; an indicator of bacterial colonization) between the treated and control group of 0.22, a standard deviation (SD) of 0.18, an α value of 0.05, and a power of 0.8 [25,26,27]. A total of 10 pure titanium disks (5 mm in diameter, Sweden Martina, Due Carrare, Italy) for each prophylactic procedure were estimated to be necessary. The following treatments were analyzed:
1) Control (no treatments).
2) Ultrasonic scaler (EMS, Nyon, Switzerland) with a conventional stainless-steel tip. Each scaling tip was angled at approximately 45° to the surface at maximum power for 10 seconds.
3) Stainless-steel Gracey curette (Medesy srl, Maniago, Italy).
4) Air-polishing device (AIRFLOW Master®, EMS) using glycine-based AIRFLOW® perio powder (AIRFLOW® powder perio, EMS). The treatment was performed at a distance of 5 mm from the disk surface for 10 seconds using the maximum settings for air and water pressure.
5) Abrasive paste for polishing (Detartrine ZTM; DZ) (Ogna, Muggio, Italy) applied with a silicon rubber cup mounted on a handpiece set at 800 rpm for 5 seconds.
After each treatment the titanium surfaces were cleaned with acetone for 1 minute, washed with distilled water, and finally air-dried. All experimental procedures were performed by the same investigator.
Surface analysis by atomic force microscopy (AFM)
After each prophylactic procedure, the disks were attached to a metal holder using a rapid-drying cyanoacrylate glue. In accordance with our previous studies [28,29,30,31], each sample was placed on an AFM (Assing, Rome, Italy), and then 20 areas (5×5 µm), representative of the entire disk surface, were randomly selected and analyzed on a 3-mm section at the tip of the disk. The AFM images of the file samples were recorded using contact mode under ambient conditions. AFM probes (curvature radius <10 nm) mounted on cantilevers (250 µm) with a spring constant of 0.1 Nm−1 were used. Three-dimensional images (400×400 lines) were processed using Gwyddion software 2.19 (http://www.gwyddion.net). The roughness average (Ra) and root mean square (RMS) of the scanned surface profiles were recorded. These parameters indicated changes in vertical surface topography, with increasing Ra and RMS values indicating alterations of the disk surfaces caused by each treatment.
Biofilm development
In these experiments, P. aeruginosa PAO1 (ATCC® BAA-47™) and S. aureus (ATCC® 25923) were used as reference strains. In addition, saliva from 3 volunteers without active caries or periodontal disease was used to isolate salivary bacteria. In particular, Streptococcus spp., Bacteroides fragilis, and Staphylococcus epidermidis were identified by biochemical assays (API®/ID32, bioMérieux, Grassina, Italy) and grown at 37°C in non-selective nutrient broth (NB; Oxoid, Basingstoke, Hants, UK). The biofilm was developed, as previously described, on sterile (gamma-irradiated) specimens in a 96-well polystyrene plate with some modifications [32]. Briefly, an overnight culture of each bacterial strain or inoculum was diluted with tryptic soy broth (TSB; BD Bioscience, Milan, Italy) to an OD600 nm of 0.2, and then incubated statically at 37°C in a humid atmosphere for 48–72 hours until a mature biofilm was obtained.
CFUs following bacterial colonization
The number of CFUs was determined by plating samples of bacterial growth from the implant surface on agar. Plates were incubated at 37°C in a humid atmosphere for 48 hours, and the number of colonies was counted.
Semi-quantitative analysis of biofilm using crystal violet staining
The amount of biofilm was determined using crystal violet staining as described by Mandrich et al. [32]. At the end of the incubation time, specimens were washed twice with sterile saline and air-dried. The adherent bacterial cells were stained with 0.1% crystal violet solution for 30 minutes. After staining, the excess crystal violet was removed and the specimens were washed 5 times with 300 μL of sterile distilled water, left to completely air-dry, and finally placed in 6-well plates containing 3 mL of 96% ethanol in order to re-solubilize the dye included in the biofilm. Crystal violet absorbance was measured at 570 nm using a microplate reader (Cytation 3, ASHI, Bernareggio, Italy). Measurements were carried out in triplicate for each disc.
Statistical analysis
Results were expressed as mean±SD. Comparisons between groups were performed using 1-way analysis of variance followed by the Tukey test for multiple comparisons. P values ≤0.05 were considered to indicate statistical significance. All data were analyzed with GraphPad Prism version 5.01 (GraphPad Inc., La Jolla, CA, USA).
RESULTS
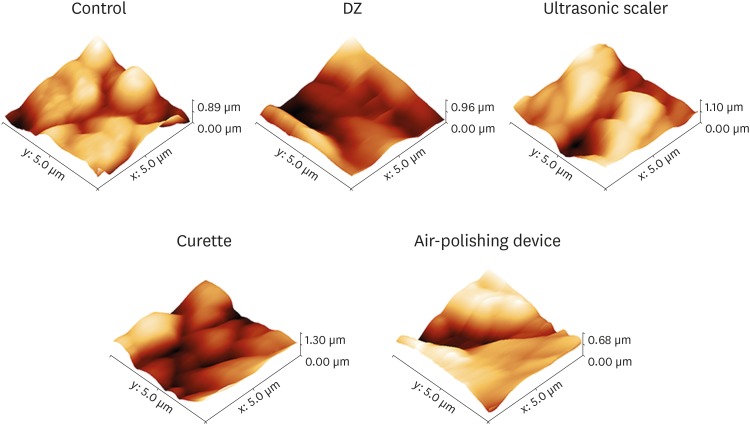
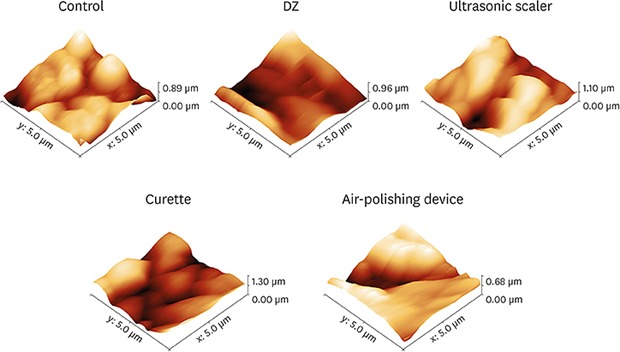
Three-dimensional AFM images of the surfaces of the treated disks and controls showed topographic irregularities at the nanometric scale (Figure 1). In particular, all treated disk surface images revealed an increase in roughness compared to the controls. To investigate the quantitative differences in topography resulting from the prophylactic procedures, vertical topographic parameters (Ra and RMS) were evaluated. The Ra and RMS values of disks treated with DZ and Gracey steel curettes were significantly lower (P<0.05) than those of the control disks (Table 1). In addition, using the ultrasonic scaler tips and the air-polishing device with glycine powder induced an increase in the mean Ra and RMS values compared to the control samples (Table 1).
Figure 1. Representative 3-dimensional atomic force microscopy images of the titanium disk surfaces.
Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette: stainless-steel Gracey curette, air-polishing device: AIRFLOW® with glycine powder.
Table 1. Mean±standard deviation of Ra and RMS values in the experimental groups (n=10).
| Variablesa) | Ra (nm) | RMS (nm) |
|---|---|---|
| Control | 134.2±8.0 | 166.7±9.8 |
| DZ | 86.2±4.1b) | 118.2±8.1b) |
| Ultrasonic scaler | 174.3±13.7 | 199.3±14.9 |
| Curette | 82.4±15.7b) | 99.2±19.0b) |
| Air-polishing device | 179.7±7.3 | 214.0±13.9 |
Ra: roughness average, RMS: root mean square, DZ: Detartrine ZTM.
a)Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette, stainless-steel Gracey curette; air-polishing device, AIRFLOW® with glycine powder; b)P<0.05: indicates statistically significant differences between the control and each prophylactic treatment.
Antibiofilm activity
Tables 2 and 3 summarize the mean, SD, sample size, P values, and results of the post hoc comparison of antibiofilm activity for all tested implants. A significant (P<0.01) reduction in biofilm formation was only found for the DZ-treated implant surfaces. In particular, DZ treatment was able to reduce the biofilm formation by about 40% for both P. aeruginosa PAO1 and S. aureus. A statistically significant reduction of S. aureus biofilm formation was also observed for DZ in comparison to the air-polishing device treatment.
Table 2. Antibiofilm activity, evaluated by a crystal violet assay, of treated implants in the presence of Pseudomonas aeruginosa PAO1, Staphylococcus aureus, Streptococcus spp., Bacteroides fragilis, and Staphylococcus epidermidis .
| Variablesa) | Pseudomonas aeruginosa PAO1 | Staphylococcus aureus | Bacteroides fragilis | Streptococcus spp. | Staphylococcus epidermidis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD570 nm (mean) | SD | No. | OD570 nm (mean) | SD | No. | OD570 nm (mean) | SD | No. | OD570 nm (mean) | SD | No. | OD570 nm (mean) | SD | No. | |
| Control | 1.36 | 0.16 | 10 | 1.20 | 0.11 | 10 | 1.20 | 0.10 | 10 | 1.20 | 0.11 | 10 | 1.16 | 0.13 | 10 |
| DZ | 1.11 | 0.13 | 10 | 0.93 | 0.04 | 10 | 0.94 | 0.07 | 10 | 0.94 | 0.06 | 10 | 0.93 | 0.07 | 10 |
| Ultrasonic scaler | 1.24 | 0.17 | 10 | 1.02 | 0.11 | 10 | 0.99 | 0.10 | 10 | 1.07 | 0.10 | 10 | 1.05 | 0.08 | 10 |
| Curette | 1.19 | 0.15 | 10 | 1.00 | 0.11 | 10 | 0.98 | 0.10 | 10 | 1.01 | 0.09 | 10 | 1.03 | 0.08 | 10 |
| Air-polishing device | 1.21 | 0.16 | 10 | 1.15 | 0.15 | 10 | 1.12 | 0.09 | 10 | 1.10 | 0.12 | 10 | 1.04 | 0.08 | 10 |
OD: optical density, SD: standard deviation, DZ: Detartrine ZTM.
a)Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette: stainless-steel Gracey curette, air-polishing device: AIRFLOW® with glycine powder.
Table 3. Results of the statistical analysis and post hoc comparison (Tukey test) between groups of antibiofilm activity.
| Test detailsa) | Pseudomonas aeruginosa PAO1 | Staphylococcus aureus | Bacteroides fragilis | Streptococcus spp. | Staphylococcus epidermidis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | |
| DZ vs. control | 0.2502 | 0.0054 | <0.01 | 0.2687 | 0.0058 | <0.01 | 0.2649 | 0.0055 | <0.01 | 0.1853 | 0.0010 | <0.01 | 0.2286 | 0.0031 | <0.01 |
| Ultrasonic scaler vs. control | 0.1213 | 0.3961 | NS | 0.1827 | 0.2148 | NS | 0.2021 | 0.2254 | NS | 0.1337 | 0.0697 | NS | 0.1084 | 0.0726 | NS |
| Curette vs. control | 0.1717 | 0.1034 | NS | 0.1928 | 0.8026 | NS | 0.2210 | 0.0879 | NS | 0.2573 | 0.1256 | NS | 0.1335 | 0.1154 | NS |
| Air-polishing device vs. control | 0.1574 | 0.1598 | NS | 0.0443 | 0.8950 | NS | 0.07935 | 0.3559 | NS | 0.09332 | 0.2257 | NS | 0.1225 | 0.2313 | NS |
| DZ vs. ultrasonic scaler | −0.1289 | 0.3354 | NS | −0.0860 | 0.4152 | NS | −0.06278 | 0.5882 | NS | −0.1236 | 0.0524 | NS | −0.1201 | 0.5363 | NS |
| DZ vs. curette | −0.07847 | 0.7775 | NS | −0.0759 | 0.5400 | NS | −0.04391 | 0.8417 | NS | −0.07208 | 0.4775 | NS | −0.09510 | 0.1476 | NS |
| DZ vs. air-polishing device | −0.09282 | 0.6533 | NS | −0.1485 | 0.0322 | <0.05 | −0.1228 | 0.0466 | <0.05 | −0.1640 | 0.1945 | NS | −0.1061 | 0.0830 | NS |
| Ultrasonic scaler vs. curette | 0.05042 | 0.9456 | NS | 0.0601 | 0.9996 | NS | 0.01886 | 0.9919 | NS | 0.05156 | 0.7645 | NS | 0.02504 | 0.9712 | NS |
| Ultrasonic scaler vs. air-polishing device | 0.03606 | 0.9838 | NS | −0.1384 | 0.0533 | NS | −0.017 | 0.8954 | NS | −0.04038 | 0.8871 | NS | 0.01409 | 0.9967 | NS |
| Curette vs. air-polishing device | −0.01436 | 0.9995 | NS | 0.0101 | 0.0546 | NS | −0.1416 | 0.0650 | NS | −0.09194 | 0.2387 | NS | −0.01095 | 0.9988 | NS |
DZ: Detartrine ZTM, NS: not significant.
a)Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette: stainless-steel Gracey curette, air-polishing device: AIRFLOW® with glycine powder.
No significant differences were found in bacterial colonization among the treated implant surfaces for any of the bacterial strains (Tables 4 and 5).
Table 4. Bacterial colonization on treated and non-treated (control) surfaces after biofilm formation.
| Variablesa) | Pseudomonas aeruginosa PAO1 | Staphylococcus aureus | Bacteroides fragilis | Streptococcus spp. | Staphylococcus epidermidis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log10 CFU (mean) | SD | No. | log10 CFU (mean) | SD | No. | log10 CFU (mean) | SD | No. | log10 CFU (mean) | SD | No. | log10 CFU (mean) | SD | No. | |
| Control | 2.00 | 0.90 | 10 | 1.99 | 0.98 | 10 | 1.98 | 1.10 | 10 | 1.99 | 0.87 | 10 | 2.01 | 1.02 | 10 |
| DZ | 1.76 | 0.58 | 10 | 1.81 | 0.95 | 10 | 1.79 | 0.65 | 10 | 1.78 | 0.89 | 10 | 1.81 | 0.89 | 10 |
| Ultrasonic scaler | 1.84 | 0.85 | 10 | 1.87 | 0.75 | 10 | 1.88 | 0.98 | 10 | 1.89 | 0.92 | 10 | 1.89 | 0.59 | 10 |
| Curette | 1.82 | 0.67 | 10 | 1.83 | 0.68 | 10 | 1.81 | 0.90 | 10 | 1.81 | 1.00 | 10 | 1.81 | 0.86 | 10 |
| Air-polishing device | 1.84 | 0.82 | 10 | 1.89 | 0.91 | 10 | 1.87 | 0.87 | 10 | 1.86 | 0.88 | 10 | 1.87 | 0.62 | 10 |
CFU: colony-forming unit, SD: standard deviation, DZ: Detartrine ZTM.
a)Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette: stainless-steel Gracey curette, air-polishing device: AIRFLOW® with glycine powder.
Table 5. Results of post hoc comparison (Tukey test) between groups of bacterial colonization.
| Test detailsa) | Pseudomonas aeruginosa PAO1 | Staphylococcus aureus | Bacteroides fragilis | Streptococcus spp. | Staphylococcus epidermidis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | Mean diff. | Adjusted P value | P | |
| DZ vs. control | 0.24 | 0.9587 | NS | 0.19 | 0.9888 | NS | 0.18 | 0.9913 | NS | 0.21 | 0.9853 | NS | 0.20 | 0.9827 | NS |
| Ultrasonic scaler vs. control | 0.16 | 0.9914 | NS | 0.12 | 0.9977 | NS | 0.10 | 0.9991 | NS | 0.10 | 0.9990 | NS | 0.12 | 0.9971 | NS |
| Curette vs. control | 0.18 | 0.9848 | NS | 0.16 | 0.9939 | NS | 0.17 | 0.9936 | NS | 0.19 | 0.9901 | NS | 0.20 | 0.9827 | NS |
| Air-polishing device vs. control | 0.16 | 0.9914 | NS | 0.099 | 0.9990 | NS | 0.11 | 0.9987 | NS | 0.13 | 0.9975 | NS | 0.14 | 0.9952 | NS |
| DZ vs. ultrasonic scaler | −0.082 | 0.9993 | NS | −0.063 | 0.9998 | NS | −0.082 | 0.9996 | NS | −0.11 | 0.9989 | NS | −0.073 | 0.9996 | NS |
| DZ vs. curette | −0.057 | 0.9998 | NS | −0.027 | >0.9999 | NS | −0.014 | >0.9999 | NS | −0.021 | >0.9999 | NS | 0.0 | >0.9999 | NS |
| DZ vs. air-polishing device | −0.082 | 0.9993 | NS | −0.086 | 0.9994 | NS | −0.070 | 0.9998 | NS | −0.078 | 0.9997 | NS | −0.056 | 0.9999 | NS |
| Ultrasonic scaler vs. curette | 0.025 | >0.9999 | NS | 0.036 | >0.9999 | NS | 0.068 | 0.9998 | NS | 0.086 | 0.9995 | NS | 0.073 | 0.9996 | NS |
| Ultrasonic scaler vs. air-polishing device | 0.0 | >0.9999 | NS | −0.023 | >0.9999 | NS | 0.012 | >0.9999 | NS | 0.029 | >0.9999 | NS | 0.017 | >0.9999 | NS |
| Curette vs. air-polishing device | −0.025 | >0.9999 | NS | −0.059 | 0.9999 | NS | −0.056 | >0.9999 | NS | −0.057 | >0.9999 | NS | −0.056 | 0.9999 | NS |
DZ: Detartrine ZTM, NS: not significant.
a)Control: pure titanium disk, DZ: abrasive paste for polishing (Detartrine ZTM) with a silicon rubber cup, ultrasonic scaler: ultrasonic scaler EMS with a conventional stainless-steel tip, curette: stainless-steel Gracey curette, air-polishing device: AIRFLOW® with glycine powder.
DISCUSSION
In this study, we evaluated the effects of mechanical treatments on titanium implants with respect to surface roughness and bacterial colonization. Plaque removal from the implant surface is a well-established technique for minimizing biofilm formation in order to prevent and treat peri-implant diseases [9,24]. Although these treatments are the procedures of choice to manage peri-implant diseases, the use of instrumentation may damage the stable protective layer of titanium oxide on the implant surface. This layer, which is a few nanometers thick, minimizes ion release from the implant to the surrounding tissues, thereby reducing inflammatory reactions in the body [33], and is responsible for antibacterial activity [34]. Therefore, any modification in the titanium oxide layer may induce implant surface corrosion, impairing cell adhesion and reducing implant biocompatibility [35].
Various modalities and instruments have been reported for the mechanical treatment of implant surfaces, but the results remain inconclusive [36]. Indeed, several instruments, such as metal curettes and conventional sonic and ultrasonic scalers, have shown to damage the implant surface severely. Conversely, non-metal instruments, including Teflon curettes, plastic instruments, and air abrasives, have been found to lead to an incomplete removal of plaque [37].
As consequence, routine prophylactic procedures causing implant surface irregularities, such as grooves and scratches, might increase the potential for plaque accumulation.
In the present study, we used AFM to evaluate the effects of different prophylactic procedures on titanium surfaces. Recently, AFM analysis was introduced to provide qualitative and quantitative information on the topography of various dental materials [29,30].
AFM reconstructs a 3-dimensional image of the surface topography in real time. These data sets can be analyzed with dedicated digital software to obtain all the relevant data pertaining to the examined surface in a quantitative form. Of additional significance, AFM allows topographic contrasts to be visualized in greater detail, enables direct measurements in all 3 dimensions with a vertical resolution of 0.1 nm (up to), and provides views of surface features in a broad range of conditions.
Two basic amplitude parameters were used in this study to characterize the implant surface roughness: Ra and RMS. These parameters were recorded for all scanned surface profiles. These parameters indicated changes in vertical surface topography, and an increase in Ra and RMS values meant alterations in the titanium instruments' surface. Implant surface roughness is closely correlated with the amount of adhering bacteria because rough surfaces have a greater contact area between the surface and bacterial cells and provide protection from shear forces [15]. Several studies reported that a Ra of 0.2 μm can be considered the threshold for obtaining a reduction of bacterial adhesion [38].
In our study, the roughness values increased in disks treated using the air-polishing device and ultrasonic scaler compared with the control group, whereas a significant reduction in the Ra and RMS values occurred when using a curette or polishing with DZ abrasive paste.
The systematic review of Louropoulou et al. [39] showed that only non-metal instruments caused minimal or no damage to both smooth and rough titanium surfaces. Furthermore, several papers reported that the use of abrasive paste, as well as rubber cups, did not induce any detrimental alterations of the surface [26].
Conversely, metal instruments resulted in severe roughening of the original smooth surface. Moreover, several in vitro and in vivo studies demonstrated that air-polishing treatments using glycine powder significantly increased the surface roughness of titanium disc surfaces, although they are considered safe and effective for biofilm removal [40].
Our results showed that both the DZ abrasive paste and stainless-steel curette significantly reduced the Ra value in comparison to the control, but the inhibition of biofilm formation was statistically significant only for DZ. Our results regarding the curette seem to be in conflict with other reports [39] indicating that curettes induced major alterations of the implant surface. However, considering that metal instruments can smoothen rough surfaces by removing the titanium surface coating [39], it can be hypothesized that the resultant destruction of the titanium oxide layer would promote bacterial colonization and biofilm formation.
In addition, a statistically significant reduction of S. aureus biofilm was observed for DZ in comparison to the air-polishing device, highlighting that this prophylactic procedure could be considered as the treatment of choice for the management of peri-implant lesions, as well as for therapy-resistant cases of periodontitis. Moreover, although we reported some differences in the CFU measurements between the instruments, no statistically significant reduction was observed, demonstrating the absence of any attachment affinity for a specific instrumented implant surface.
In conclusion, this study demonstrated that prophylactic procedures altered the roughness of titanium disks, influencing their antibiofilm properties, and DZ abrasive paste was found to be the treatment of choice for the management of peri-implant lesions, as well as for therapy-resistant cases of periodontitis. However, considering the limitations of this in vitro study, it may be necessary to obtain more robust scientific evidence to support the choice of a prophylactic procedure in the treatment of periodontitis and peri-implant diseases.
Footnotes
Funding: This work was supported by POR FESR 2014-2020 – Regione Campania, Asse 1 – obiettivo specifico 1.2, Progetto “Sviluppo di nanotecnologie Orientate alla Rigenerazione e Ricostruzione tissutale, Implantologia e Sensoristica in Odontoiatria/oculistica (SORRISO)” - pdt1-000410.
- Conceptualization: Anna Di Salle, Gianrico Spagnuolo, Gianfranco Peluso, Carlo Rengo.
- Formal analysis: Anna Di Salle, Gianrico Spagnuolo, Gianfranco Peluso.
- Investigation: Anna Di Salle, Gianrico Spagnuolo, Raffaele Conte, Alfredo Procino.
- Methodology: Anna Di Salle, Gianrico Spagnuolo, Raffaele Conte, Alfredo Procino, Gianfranco Peluso, Carlo Rengo.
- Project administration: Gianrico Spagnuolo, Carlo Rengo.
- Writing - original draft: Anna Di Salle, Gianrico Spagnuolo, Gianfranco Peluso.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017;96:31–37. doi: 10.1177/0022034516667484. [DOI] [PubMed] [Google Scholar]
- 2.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 3.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(Suppl):286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T, Buser D, Chen ST, Cochran D, DeBruyn H, Jemt T, et al. Statements from the Estepona consensus meeting on peri-implantitis, February 2–4, 2012. Clin Implant Dent Relat Res. 2012;14:781–782. doi: 10.1111/cid.12017. [DOI] [PubMed] [Google Scholar]
- 5.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 7.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 8.Chenicheri S, R U, Ramachandran R, Thomas V, Wood A. Insight into oral biofilm: primary, secondary and residual caries and phyto-challenged solutions. Open Dent J. 2017;11:312–333. doi: 10.2174/1874210601711010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl 16):S152–S157. doi: 10.1111/jcpe.12369. [DOI] [PubMed] [Google Scholar]
- 10.Salvi GE, Ramseier CA. Efficacy of patient-administered mechanical and/or chemical plaque control protocols in the management of peri-implant mucositis. A systematic review. J Clin Periodontol. 2015;42(Suppl 16):S187–S201. doi: 10.1111/jcpe.12321. [DOI] [PubMed] [Google Scholar]
- 11.Larsen T, Fiehn NE. Dental biofilm infections - an update. APMIS. 2017;125:376–384. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 12.Lin NJ. Biofilm over teeth and restorations: what do we need to know? Dent Mater. 2017;33:667–680. doi: 10.1016/j.dental.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009;24:616–626. [PubMed] [Google Scholar]
- 14.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 15.Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 2015;94:1027–1034. doi: 10.1177/0022034515587690. [DOI] [PubMed] [Google Scholar]
- 16.Suárez-López Del Amo F, Yu SH, Wang HL. Non-surgical therapy for peri-implant diseases: a systematic review. J Oral Maxillofac Res. 2016;7:e13. doi: 10.5037/jomr.2016.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraris S, Spriano S. Antibacterial titanium surfaces for medical implants. Mater Sci Eng C. 2016;61:965–978. doi: 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Kreisler M, Kohnen W, Christoffers AB, Götz H, Jansen B, Duschner H, et al. In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er: YAG laser and an air powder system. Clin Oral Implants Res. 2005;16:36–43. doi: 10.1111/j.1600-0501.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater. 2009;88:83–91. doi: 10.1002/jbm.b.31154. [DOI] [PubMed] [Google Scholar]
- 20.Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011;38:872–878. doi: 10.1111/j.1600-051X.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 21.Albertini M, López-Cerero L, O'Sullivan MG, Chereguini CF, Ballesta S, Ríos V, et al. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin Oral Implants Res. 2015;26:937–941. doi: 10.1111/clr.12387. [DOI] [PubMed] [Google Scholar]
- 22.Canullo L, Rossetti PH, Penarrocha D. Identification of Enterococcus faecalis and Pseudomonas aeruginosa on and in implants in individuals with peri-implant disease: a cross-sectional study. Int J Oral Maxillofac Implants. 2015;30:583–587. doi: 10.11607/jomi.3946. [DOI] [PubMed] [Google Scholar]
- 23.Harris LG, Mead L, Müller-Oberländer E, Richards RG. Bacteria and cell cytocompatibility studies on coated medical grade titanium surfaces. J Biomed Mater Res A. 2006;78:50–58. doi: 10.1002/jbm.a.30611. [DOI] [PubMed] [Google Scholar]
- 24.Renvert S, Lindahl C, Renvert H, Persson GR. Clinical and microbiological analysis of subjects treated with Brånemark or AstraTech implants: a 7-year follow-up study. Clin Oral Implants Res. 2008;19:342–347. doi: 10.1111/j.1600-0501.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehl C, Kern M, Zimmermann A, Harder S, Huth S, Selhuber-Unkel C. Impact of cleaning procedures on adhesion of living cells to three abutment materials. Int J Oral Maxillofac Implants. 2017;32:976–984. doi: 10.11607/jomi.5630. [DOI] [PubMed] [Google Scholar]
- 26.Cafiero C, Aglietta M, Iorio-Siciliano V, Salvi GE, Blasi A, Matarasso S. Implant surface roughness alterations induced by different prophylactic procedures: an in vitro study. Clin Oral Implants Res. 2017;28:e16–20. doi: 10.1111/clr.12849. [DOI] [PubMed] [Google Scholar]
- 27.Chen CJ, Ding SJ, Chen CC. Effects of surface conditions of titanium dental implants on bacterial adhesion. Photomed Laser Surg. 2016;34:379–388. doi: 10.1089/pho.2016.4103. [DOI] [PubMed] [Google Scholar]
- 28.Ametrano G, D’Antò V, Di Caprio MP, Simeone M, Rengo S, Spagnuolo G. Effects of sodium hypochlorite and ethylenediaminetetraacetic acid on rotary nickel-titanium instruments evaluated using atomic force microscopy. Int Endod J. 2011;44:203–209. doi: 10.1111/j.1365-2591.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 29.Spagnuolo G, Ametrano G, D’Antò V, Rengo C, Simeone M, Riccitiello F, et al. Effect of autoclaving on the surfaces of TiN-coated and conventional nickel-titanium rotary instruments. Int Endod J. 2012;45:1148–1155. doi: 10.1111/j.1365-2591.2012.02088.x. [DOI] [PubMed] [Google Scholar]
- 30.D'Antò V, Rongo R, Ametrano G, Spagnuolo G, Manzo P, Martina R, et al. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012;82:922–928. doi: 10.2319/100211-620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rongo R, Ametrano G, Gloria A, Spagnuolo G, Galeotti A, Paduano S, et al. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2014;84:665–672. doi: 10.2319/081213-593.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandrich L, Cerreta M, Manco G. An engineered version of human PON2 opens the way to understand the role of its post-translational modifications in modulating catalytic activity. PLoS One. 2015;10:e0144579. doi: 10.1371/journal.pone.0144579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillemot F, Prima F, Tokarev VN, Belin C, Porté-Durrieu MC, Gloriant T, et al. Ultraviolet laser surface treatment for biomedical applications of β titanium alloys: morphological and structural characterization. Appl Phys, A Mater Sci Process. 2003;77:899–904. [Google Scholar]
- 34.Gallardo-Moreno AM, Pacha-Olivenza MA, Fernández-Calderón MC, Pérez-Giraldo C, Bruque JM, González-Martín ML. Bactericidal behaviour of Ti6Al4V surfaces after exposure to UV-C light. Biomaterials. 2010;31:5159–5168. doi: 10.1016/j.biomaterials.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Fox SC, Moriarty JD, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990;61:485–490. doi: 10.1902/jop.1990.61.8.485. [DOI] [PubMed] [Google Scholar]
- 36.Mengel R, Buns CE, Mengel C, Flores-de-Jacoby L. An in vitro study of the treatment of implant surfaces with different instruments. Int J Oral Maxillofac Implants. 1998;13:91–96. [PubMed] [Google Scholar]
- 37.Hallmon WW, Waldrop TC, Meffert RM, Wade BW. A comparative study of the effects of metallic, nonmetallic, and sonic instrumentation on titanium abutment surfaces. Int J Oral Maxillofac Implants. 1996;11:96–100. [PubMed] [Google Scholar]
- 38.Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11:169–178. [PubMed] [Google Scholar]
- 39.Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23:643–658. doi: 10.1111/j.1600-0501.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 40.Bennani V, Hwang L, Tawse-Smith A, Dias GJ, Cannon RD. Effect of air-polishing on titanium surfaces, biofilm removal, and biocompatibility: a pilot study. BioMed Res Int. 2015;2015:491047. doi: 10.1155/2015/491047. [DOI] [PMC free article] [PubMed] [Google Scholar]