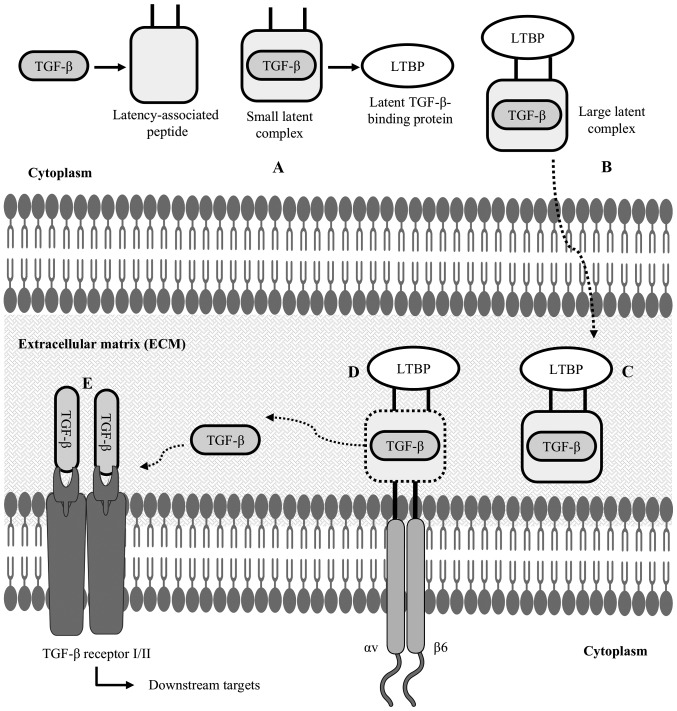
Figure 3.
Latent TGF-β structure and activation of TGF-β: (A) after synthesizing TGF-β inside the cytoplasm of a cell, LAP forms a straightjacket around the TGF-β, resulting in a small latent complex; (B) this small latent complex binds to LTBP to form a LLC; (C) this is an inactive state of TGF-β, which is now secreted in the ECM; (D) in the ECM, cell-associated αvβ6 integrin binds to the arginyl-glycyl-aspartic acid (RGD) domain of the latency-associated peptide, cleaving the LTBP interaction; (E) TGF-β is then released from the LAP, allowing it to interact with its receptor and activate TGF-β-mediated downstream targets. LAP, latency-associated peptide; LTBP, latent TGF-β-binding protein; LLC, large latent complex; ECM, extracellular matrix.