Abstract
Objective:
Τo investigate the effect of NF-kB signaling pathway on the expression of MIF, TNF-α, and IL-6 in the regulation of disc degeneration.
Methods:
The disc tissue was taken from 56 patients with cervical spondylosis. According to the preoperative MRI and intraoperative disc herniation, the patients were divided into two groups: degeneration group and herniation group. The control group was 34 patients with cervical trauma with no history of cervical spondylosis. According to the preoperative JOA scores of cervical spondylosis, patients were divided into three groups: mild, moderate and severe. ELISA was used to detect the expression of MIF, IL-6, and TNF-α in the cervical intervertebral disc. NF-kB mRNA expression in the intervertebral disc was detected by qRT-PCR.
Results:
The expression levels of NF-kB mRNA, MIF, IL-6 and TNF-α in the control group were significantly higher than those in the degeneration group and the herniation group (p<0.05). There was a positive correlation between the expression of NF-kB mRNA, MIF, IL-6, TNF- and cervical intervertebral disc degeneration. The expression of MIF, IL-6, and TNF-α in the mild, moderate, and severe group was negatively correlated with the JOA score.
Conclusions:
The expressions of NF-kB, MIF, IL-6, and TNF-α in intervertebral disc tissue in patients with disc herniation were increased and related to the degree of disc herniation. It may play an important role in the pathophysiological process of disc herniation.
Keywords: NF-Kb, MIF, TNF-α, IL-6, Intervertebral Disc Degeneration
Introduction
Intervertebral disc degeneration (IDD) is the premise and pathological basis of various degenerative diseases of the spine. It is a chronic and gradual pathological and physiological processes with mainly low back pain and cervical pain[1]. IDD is one of the clinically common diseases. Some studies have shown that[2] IDD patients are mostly male, and 70% of patients have a history of waist disease, and IDD is more frequent in middle-aged and old patients. However, in recent years, there have been reports of increased numbers of young and middle-aged patients. This indicates that the onset age of patients is getting younger and younger, reducing the national labor force and productivity and increasing economic losses. Normal intervertebral discs have a large elasticity and can withstand a lot of pressure. However, with age, they are affected by the external forces such as twisting and squeezing, slight injury occurs. Accumulation of long years causes the rupture of the annulus fibrosis, leading to the occurrence of IDD. At present, the treatment for IDD is mainly divided into two types of treatment: surgical treatment and conservative treatment[3,4].
Nuclear factor-kappa B (NF-kB) is a unique nuclear transcription factor that is widely present in the cytoplasm of higher eukaryotes. NF-kB is mostly present in the form of P65-P50 dimer and is associated with inhibitor IkB binds in an inactive state[5]. Studies have shown that[6] the persistently activated NF-kB pathway is involved in a variety of inflammatory diseases. However, IDD is closely related to the autoimmune inflammatory response mediated by various inflammatory cytokines (IL-1, IL-6). Some studies have shown that the expression of NF-kB p65 is significantly enhanced during the induction of IDD by proinflammatory cytokines[7,8]. Macrophage migration inhibitory factor (MIF) is a pro-inflammatory factor that mobilizes macrophages and plays a specific inhibitory effect on the release of glucocorticoids[9].
Therefore, this study examined the role of NF-kB signaling pathway and cytokines in the mechanism and regulation of IDD.
General data and methods
Sample selection and patient data
This study collected 56 cases of lumbar intervertebral discs of cervical spondylosis patients from June 2015 to December 2016 in Shanghai Songjiang District Central Hospital. There were 33 male patients and 23 female patients. The age range of the patients ranged from 37 to 66 years, and the average age was 53.4±11.5 years. According to preoperative MRI conditions and intraoperative disc status, 56 patients were divided into degenerative group (n=29) and herniation group (n=27); another 34 patients with cervical trauma but without cervical spondylosis were included, including 18 male patients and 16 female patients, age range 27-40 years, mean age 35.62±5.43 years; patients were divided into three groups according to the preoperative JOA score of cervical spondylosis: mild group (18 cases), moderate group (26 cases) and severe group (12 cases). The study was approved by the Medical Ethics Committee of Shanghai Songjiang District Central Hospital. All patients were informed and signed informed consent. Patient clinical data are shown in [Table I].
Table I.
Clinical Data [n(%)].
| Degeneration group (n=29) | Herniation group (n=27) | Control group (n=34) | |
|---|---|---|---|
| Gender | |||
| Male | 18(62.1) | 15(55.56) | 18(60.00) |
| Female | 11(37.9) | 12(44.44) | 16(40.00) |
| Age (year) | 56.15±6.42 | 52.36±4.25 | 35.62±5.43 |
| BMI (kg/m2) | 21.57±1.54 | 22.1±1.83 | 21.33±0.86 |
| Herniation degeneration position/trauma position | 6.783 | 0.560 | |
| C3,4 intervertebral disc | 5(17.24) | 6(22.22) | 3(10.00) |
| C4,5 intervertebral disc | 10(34.48) | 9(33.33) | 6(20.00) |
| C5,6 intervertebral disc | 8(27.59) | 8(29.63) | 14(46.67) |
| C6,7 intervertebral disc | 5(17.24) | 3(11.11) | 7(23.33) |
| C7, T1 disc | 1(3.45) | 1(3.71) | 0(0.00) |
| JOA Score | |||
| Mild group ≥13 points | 12(41.38) | 11(40.74) | |
| Moderate group 9~12 points | 12(41.38) | 10(37.04) | |
| Severe group ≤8 points | 5(17.24) | 6(22.22) | |
Inclusion and exclusion criteria
Inclusion criteria: The patient developed lumbar, cervical spine pain, and the duration of illness exceeded 6 months; the patient had not undergone surgery before the hospital admission examination; the patient’s imaging examination revealed a herniated intervertebral disc, and the discography was positive.
Exclusion criteria: Patients have congenital defects, disability, family genetic history; patients with hypertension, diabetes; patients with impaired immune function; patients who do not cooperate with the examination, do not cooperate with the treatment.
Main reagents and instruments
TRIzol reagents, PCR kits, and reverse transcription kits were purchased from Invitrogen, IL-1, and IL-6. MIF Elisa kits were purchased from Thermo Scientific, USA, and Lowry protein concentration kits were purchased from Biotime Institute of Biotechnology, ABI Prism 7900 PCR instrument was purchased from ABI, USA. NF-kB mRNA was designed and synthesized by Shanghai Sangon Biotechnology Co., Ltd.
Experimental method
Patient specimen collection
In this study, all patients underwent anterior cervical discectomy and fusion and fixation. The fresh specimens taken out were washed with phosphate buffer for several times, and the blood adsorbed on the tissues was washed. After flushing, the specimens were stored in liquid nitrogen within 15 to 30 minutes, and the subsequent experiments were performed in time.
Experiment
The specimens were collected for nucleus pulposus tissue extraction. Each 350 mg of nucleus pulposus tissue was added with 1 mL of NaCl (0.9%) and ground in a homogenate tube to make a suspension. The sample was centrifuged at 3500 r/min for 3 minutes at a speed of 3500 r/min. The supernatant was extracted by centrifugation. The sample dilution was determined and loaded, MIF, TNF-α, IL-6 expression levels were detected according to the kit instructions, the sample was diluted 10 times with the kit calibration diluent, and the 50L mixture was added to the microporous plate wells and then incubated for 2 hours. After incubation, wash and add the detection antibody for 2 h. After washing again, the substrate solution was incubated for 30 min in dark and light was developed. Finally, the termination solution was added, and the optical density was measured at 450 nm. The optical densities of the samples were interpolated using the MIF, TNF-α, and IL-6 standards generated in the kit and the concentrations calculated. The protein concentration was determined using the Lowry method.
PCR detection
The nucleus pulposus tissue was ground and added to the lysate. TRIzol reagent was used to extract total RNA. After extraction, the concentration and purity of total RNA were identified using 1% agarose gel and UV spectrophotometer. Reverse transcription of total RNA was performed according to the cDNA kit instructions. A portion of the cDNA product was subjected to subsequent experiments, and the excess product was stored at -20° C until use. ABI Prism 7900 PCR instrument was used for PCR amplification, PCR system: PCR mix (2XTamix) 12.5L, DNA template 2.0L, 1.0L each of upstream and downstream primers, and double distilled water to make up to 25L. PCR reaction conditions: 95° C for 5 min, 95° C for 30 s, 60° C for 30 s, 72° C for 1 min. A total of 40 amplifications were performed at this time, and the final extension was at 72° C for 5 min. GAPDH was used as an internal reference and the experiment was conducted 3 times in total.
Statistical method
This experiment uses SPSS20.0 statistical software to carry out data collation analysis on the collected data. The measurement data in the text was expressed as the mean ± standard deviation, (x±S), the enumeration data were expressed as a percentage (%), and the average between the two groups was compared with the t-test. The chi-square test was used to analyze the enumeration data. P<0.05 for the difference was statistically significant.
Results
NF-kB relative expression level
We detected the relative expression levels of NF-kB in the degenerative group, the herniation group, and the control group, and found that there was a statistically significant difference in the relative expression level of NF-kB among the groups (F=278.652, p=0.001). The relative expression level of NF-kB in the nucleus pulposus of patients in the degeneration group was significantly higher than that in the control group. There was a statistically significant difference between the two groups (t=19.672, p=0.001). The relative expression level of NF-kb in the degenerative group was significantly lower than compared with the herniation group, the difference was statistically significant (t=4.131, p=0.001), the expression level of NF-kB in the herniation group was significantly higher than that of the control group (t=24.590, p=0.001) (Figure 1).
Figure 1.
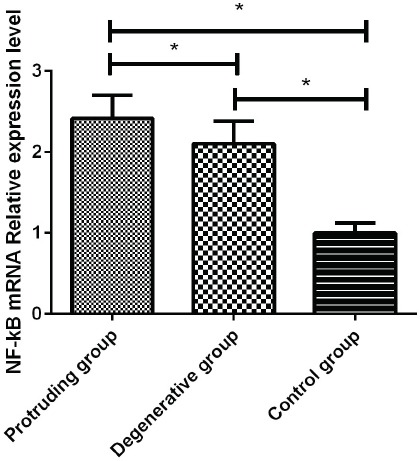
The expression of NF-kB mRNA. The expression of NF-Kb mRNA in the herniation group, degeneration group and control group was detected by qRT-PCR method. The expression of NF-Kb was significantly higher in the herniation group than in the other two groups, the difference was statistically significant (p<0.05). The expression of NF-kB mRNA in the degeneration group was higher than that in the control group (p<0.05). *Statistical difference between the two groups (p<0.05).
MIF, TNF-α, IL-6 expression levels in patients
The expressions of MIF, TNF-α and IL-6 in the degenerative group, the herniation group, and the control group were detected. The expressions of MIF, TNF-α and IL-6 in the degenerative group and the herniation group were higher than the control group, the difference was significantly statistically significant (p<0.01). By comparing the expression levels of MIF, TNF-α, and IL-6 in the degenerative group and the herniation group, it was found that the expression level in the degenerative group was lower than that of the herniation group, there is a difference between the two groups (Figure 2).
Figure 2.
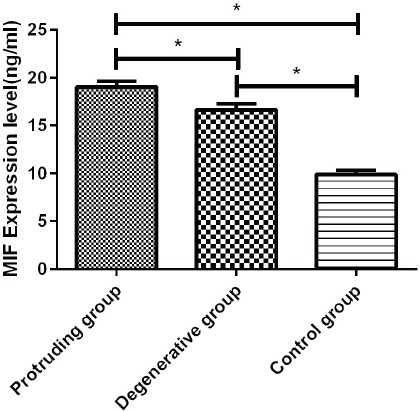
Expression of MIF, TNF-α, and IL-6 protein. Expression of MIF, TNF-α and IL-6 in the herniation group, degeneration group, and control group was detected by ELISA. The expression of the MIF, TNF-α, and IL-6 in the herniation group was significantly increased in comparison to the other two groups (p<0.05), the expression of MIF, TNF-α and IL-6 protein in the degeneration group was higher than that in the control group (p<0.05). *Statistical difference between the two groups (p<0.05).
Comparison of the expression of MIF, TNF-α, IL-6 and NF-kB in three groups patients with mild, moderate and severe JOA score
According to the JOA score, the degeneration and herniation group of patients were divided mild, moderate and severe groups to compare the levels of MIF, TNF-α, IL-6 and NF-kB. The results showed that the expression of MIF, TNF-α, IL-6 and NF-kB gradually increased with the increase of JOA score in three groups, and there were statistical differences between the groups (p<0.01). The expression level of the severe group was significantly higher than that of the control group and the degeneration group, the difference was significant (p<0.05). There was a significant increase in degeneration group compared to the control group (p<0.05). (Figure 3) The relationship between MIF, TNF-α, IL-6, and NF-kB in degenerative patients.
Figure 3.
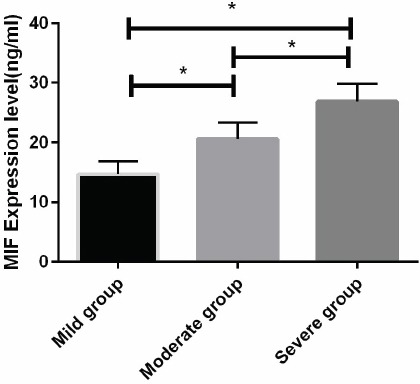
Expression of NF-kB mRNA, MIF, TNF- α and IL-6 in patients with mild, moderate, and severe JOA scores We detected NF-kB mRNA, MIF, and TNF- α in each group by qRT-PCR and ELISA. IL-6 protein, NF-kB, MIF, TNF-α, IL-6 expression levels in all groups increased significantly with the decrease of JOA score. * Statistical difference between the two groups (p<0.05).
We performed a Pearson correlation analysis of the relationship between MIF, TNF-α, IL-6, and NF-kB in degenerative patients and found that the expression levels of MIF, TNF-α and IL-6 were positively related to NF-kB, that is, with the increase of the expression of MIF, TNF-α and IL-6, the relative expression of NF-Kb was also increased (Table II).
Table II.
The relationship between MIF, TNF-α, IL-6 and NF-kB in the degenerative group.
| Group | r | P |
|---|---|---|
| MIF | 0.567 | 0.011 |
| TNF-α | 0.689 | 0.015 |
| IL-6 | 0.598 | 0.043 |
Discussion
IDD is a degenerative disease caused by the aging of disc tissue such as nucleus pulposus, cartilage endplates, and annulus fibrosus with increasing age[10]. The characteristic change of IDD is that the aging of the nucleus pulposus is accompanied by a decrease in a large number of proteoglycans and a loss of water[11]. An autopsy found IDD in more than 80% of adults. A large number of surveys have shown[12] that more than 85% of patients worldwide are admitted to hospital because of IDD-induced diseases, and more than half of them are middle-aged and elderly people. With the accelerated aging of the population, the incidence of multiple diseases caused by IDD has increased. This has affected people’s quality of life and aggravated their economic burden. With the development of clinical research and imaging technology in recent years, there is increasing evidence that inflammatory factors play a key regulatory role in the development and pathological processes of IDD[13].
NF-kB is an important regulator of cellular gene transcription and can regulate various cytokines or receptors, thereby promoting or inhibiting the expression of chemotaxis and related apoptotic proteins and regulating the body[14]. Studies have shown[15] that when cells are induced by inflammatory mediators or complement, oxidative stress and other factors, NF-kB inhibitor, IKB, dissociates, leading to NF-kB exposure through the cytoplasm into the nucleus to accelerate DNA transcription, the expression of cytokines and inflammatory mediators is regulated so as to regulate the physiological and pathological processes of tissues and cells. Through JOA scores, the expression of NF-kB in patients was found. However, it is not clear whether the NF-kB pathway activates or inhibits which inflammatory factors. Therefore, we tested its target genes downstream, MIF is an active protein secreted by activated T cells, and can also be largely secreted by the pituitary and monocyte-macrophages themselves in addition to T cells[16]. As an important pro-inflammatory factor, it has been used to inhibit the anti-inflammatory effects of glucocorticoids, thereby releasing a large number of inflammatory factors. In recent years, studies have shown that[17] MIF is associated with a variety of immune and inflammatory diseases and that MIF can be used as a potential target for the treatment of inflammatory and immune diseases through in vitro and in vivo experiments. In this study, we found that with the JOA group, the expression of MIF was significantly increased with the decrease of JOA during the experiment.
As a multi-functional cytokine, TNF-α is secreted in large amounts by monocytes and macrophages, which can reduce the synthesis of both proteoglycans and collagens, thereby reducing their synthesis[18]. The process of the development of IL-6IDD is closely linked to the secretion of bone marrow stromal cells, monocytes, and macrophages[7]. Studies have shown that[19] IL-6 has a duality of both pro-inflammatory and anti-inflammatory effects. In this study, we found that with the increase in the expression of IL-6, the patient’s condition was more severe. During the experiment, the expression of IL-6 was significantly increased with the decrease of JOA according to the JOA grouping.
Based on the above, we speculated that during the IDD process, activation of NF-kB pathway, activation of NF-kB into the nucleus and DNA for transcription induces the release of its downstream target gene MIF, etc., which in turn causes a large number of inhibitory effects of glucocorticoids on MIF. The release of IL-6 and TNF-α. In this study, we detected nucleus pulposus tissue in patients with disc herniation and intervertebral disc degeneration and found that the relative expression levels of NF-kB and the expression levels of MIF, TNF-α, and IL-6 in the herniation group were significantly higher than those in the degenerative group. All of them are higher than the control group. This well proves our hypothesis. At the end of the study, we performed a correlation analysis and found that the expression of MIF, TNF-α, and IL-6 was positively correlated with the expression of NF-kB. It is well proved that the NF-kB pathway regulates downstream target genes MIF, TNF-α, and IL-6 through positive feedback. However, in the herniation group, the expression level was higher than that in the degenerative group. We speculate that the abnormal activation of the NF-kB pathway may accelerate the patient’s pathological changes under the influence of many factors.
However, there are still some defects in this study. First of all, we have a small sample size. We have not yet confirmed whether this result is biased. Secondly, the samples we collected this time are cervical vertebrae samples. The unitary nature of the specimens may affect our results. Therefore, in the future research, we hope to further improve our research results by increasing the number and types of our samples and to corroborate the correctness of our results.
In summary, the expression of NF-kB, MIF, IL-6, TNF-α is increased in degenerated disc tissue of patients with disc herniation and is associated with the degree of disc herniation. It may play an important role in the pathophysiological process of disc herniation.
Authors’ contributions
HL and XY drafted this manuscript. HL, XY and CL were mainly devoted to collecting and interpreting the general data. HL and ZS detected MIF, TNF-α, IL-6 expression levels. XY and XW were responsible for PCR. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Songjiang District Central Hospital. Signed written informed consents were obtained from the patients.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration:pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosley GE, Evashwick-Rogler TW, Lai A, Iatridis JC. Looking beyond the intervertebral disc:the need for behavioral assays in models of discogenic pain. Ann N Y Acad Sci. 2017;1409(1):51–66. doi: 10.1111/nyas.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gullbrand SE, Malhotra NR, Schaer TP, Zawacki Z, Martin JT, Bendigo JR, Milby AH, Dodge GR, Vresilovic EJ, Elliott DM, et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis Cartilage. 2017;25(1):146–156. doi: 10.1016/j.joca.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Garcia O, Matsuzaki T, Olmer M, Masuda K, and Lotz MK. Age-related reduction in the expression of FOXO transcription factors and correlations with intervertebral disc degeneration. J Orthop Res. 2017;35(12):2682–2691. doi: 10.1002/jor.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas DK, Dai SC, Cruz A, Weiser B, Graner E, Pardee AB. The nuclear factor kappa B (NF-kappa B):a potential therapeutic target for estrogen receptor negative breast cancers. Proc Natl Acad Sci U S A. 2001;98(18):10386–91. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman A, Kokkonen H, Arlestig L, Berglin E, Rantapaa-Dahlqvist S. Receptor activator of nuclear factor kappa-B ligand (RANKL) but not sclerostin or gene polymorphisms is related to joint destruction in early rheumatoid arthritis. Clin Rheumatol. 2017;36(5):1005–1012. doi: 10.1007/s10067-017-3570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–57. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 8.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, Pear WS, Hatton RD, and Weaver CT. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance. Nat Immunol. 2015;16(3):286–95. doi: 10.1038/ni.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israelson A, Ditsworth D, Sun S, Song S, Liang J, Hruska-Plochan M, McAlonis-Downes M, Abu-Hamad S, Zoltsman G, Shani T, et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron. 2015;86(1):218–32. doi: 10.1016/j.neuron.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Z, Sun H, Xie B, Xia D, Zhang X, Yu D, Li J, Xu Y, Wang Z, Wu Y, et al. Therapeutic effects of gefitinib-encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration. Biomaterials. 2018;160:56–68. doi: 10.1016/j.biomaterials.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Hansen T, Smolders LA, Tryfonidou MA, Meij BP, Vernooij JCM, Bergknut N, and Grinwis GCM. The Myth of Fibroid Degeneration in the Canine Intervertebral Disc:A Histopathological Comparison of Intervertebral Disc Degeneration in Chondrodystrophic and Nonchondrodystrophic Dogs. Vet Pathol. 2017;54(6):945–952. doi: 10.1177/0300985817726834. [DOI] [PubMed] [Google Scholar]
- 12.Sakai D, and Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–71. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Tian Y, Wang J, Phillips KLE, Binch ALA, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, et al. Inflammatory Cytokines Induce NOTCH Signaling in Nucleus Pulposus Cells:implications in intervertebral disc degeneration. J Biol Chem. 2013;288(23):16761–74. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, and Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One. 2015;10(3):e0122821. doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Sun Z, Wang S, Li Z, and Zheng L. Wnt1 Participates in Inflammation Induced by Lipopolysaccharide Through Upregulating Scavenger Receptor A and NF-kB. Inflammation. 2015;38(4):1700–6. doi: 10.1007/s10753-015-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dambacher J, Staudinger T, Seiderer J, Sisic Z, Schnitzler F, Pfennig S, Hofbauer K, Konrad A, Tillack C, Otte JM, et al. Macrophage migration inhibitory factor (MIF) -173G/C promoter polymorphism influences upper gastrointestinal tract involvement and disease activity in patients with Crohn's disease. Inflamm Bowel Dis. 2007;13(1):71–82. doi: 10.1002/ibd.20008. [DOI] [PubMed] [Google Scholar]
- 17.Al-Abed Y, and VanPatten S. MIF as a disease target:ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3(1):45–63. doi: 10.4155/fmc.10.281. [DOI] [PubMed] [Google Scholar]
- 18.Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, Loebstein R, Chowers Y, Eliakim R, Kopylov U, et al. Optimizing Anti-TNF-alpha Therapy:Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2016;14(4):550–557. e2. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Salama AD, Little MA. The Janus Faces of IL-6 in GN. J Am Soc Nephrol. 2015;26(7):1480–2. doi: 10.1681/ASN.2014111141. [DOI] [PMC free article] [PubMed] [Google Scholar]


