Abstract
Regulatory effect of puerarin on bladder cancer T24-cell apoptosis and its possible mechanism were investigated. The experimental subjects were divided into the experimental group, the control group and the blank control group, and the cell inhibition rates after treatment were detected, respectively. Then, subjects were further divided into the control group, the puerarin group (treated with puerarin), the agonist group [treated with silent information regulator 1 (SIRT1) agonist SRT1720], the inhibitor group (treated with SIRT1 inhibitor EX527) and the combination group (treated with SRT1720, and then with puerarin). Apoptosis in each group was detected via flow cytometry, and the expression of apoptosis-related proteins, and SIRT1 and p53 proteins in each group was detected via western blotting. Moreover, the expression of SIRT1 and p53 messenger ribonucleic acid (mRNA) was detected via reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The inhibition rate of bladder cancer T24 cells was significantly increased after treatment with puerarin at different concentrations. Compared with those in the normal control group, the inhibition rates at 24, 48 and 72 h after treatment with puerarin were significantly increased (p<0.05). Compared with those in the control group, the apoptosis rate of T24 cells was remarkably increased after treatment with different doses of puerarin or EX527, and the expression level of apoptosis-related protein Bcl-2-associated X protein (Bax) was also significantly increased, but the expression level of B-cell lymphoma 2 (Bcl-2) was decreased, and both the protein and mRNA expression levels of SIRT1 and p53 also significantly declined. Compared with those in the puerarin group, the apoptosis rate in the combination group was decreased, and the expression level of apoptosis-related protein Bax was also significantly decreased, but the expression level of Bcl-2 was increased, and SIRT1 and p53 protein expression levels were also remarkably increased. Puerarin can inhibit the proliferation of bladder cancer T24 cells and induce apoptosis, and the possible mechanism is related to the inhibition of SIRT1/p53 signaling pathway.
Keywords: puerarin, bladder cancer, silent information regulator 1, p53, apoptosis
Introduction
Bladder cancer is one of the most common malignant tumors in urinary surgery, and the incidence rate shows a continuously increasing trend in the world (1). In China, the morbidity and mortality rates of bladder cancer rank first in malignant tumors of the male reproductive system (2). Due to the lack of early diagnostic means, lymph node metastasis has often occurred at the diagnosis of disease. At present, surgical treatment is mainly adopted for bladder cancer in clinical practice, but such a method is more traumatic to patients. As a result, the quality of life is not high, and tumor cells are prone to metastasis (3). In addition to surgery, conventional basic chemotherapy drugs, such as cisplatin, are also one of the clinical treatment methods, which can achieve satisfactory effects within a short period. Due to the longer cycle of chemotherapy, however, the patient's body is prone to drug tolerance, and bone marrow suppression and adverse reactions in the nervous system occur easily (4). Therefore, searching for an effective and safe drug is currently a main direction in the clinical treatment of bladder cancer.
Puerarin is an isoflavone substance extracted from plants such as Pueraria thomsonii, and plays an important role in tumor apoptosis, proliferation and immunity (5). Studies have shown that puerarin can induce apoptosis of human cervical cancer cells through inhibiting Wnt/β-catenin signaling pathway (6). Numerous studies have confirmed that puerarin has a significant inhibitory effect on various malignant tumor cells, including bladder cancer cells (7). However, there is little research on puerarin in regulating bladder cancer cell proliferation and apoptosis, and its possible molecular mechanism remains unclear. Silent information regulator 1 (SIRT1) is one of the members of the mammalian sirtuin family with the main role of regulating the substance metabolism and lifespan (8). According to studies, SIRT1 can deacetylate the lysine residue at position 382 of the tumor suppressor protein p53, thereby reducing p53 activity and allowing cells to bypass p53-mediated apoptosis and continue to survive (9). However, little is known about the role of SIRT1 in bladder cancer cells. In this study, the regulatory effects of puerarin on apoptosis-related proteins, SIRT1 and p53 in human bladder cancer T24 cells were observed, and the effect of puerarin on apoptosis of bladder cancer cells and its molecular mechanism were preliminarily studied.
Materials and methods
Materials
Cell lines
Bladder cancer T24 cell lines (cat. no. FS-0139) were purchased from the Cell Bank of the Peking Union Medical College.
Main reagents
High-glucose Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and trypsin were purchased from Dow Corning (Midland, MI, USA). Methyl thiazolyl tetrazolium (MTT) kit and Annexin V cell apoptosis kit were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). M-MuLV Reverse Transcriptase kit and SYBR-Green I real-time fluorescence quantitative polymerase chain reaction (PCR) kit were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). SIRT1 agonist SRT1720 and inhibitor EX527 were from Sigma-Aldrich (Sigma-Aldrich: Merck KGaA, St. Louis, MO, USA) [dissolved at 1 mmol/l with dimethylsulfoxide (DMSO), and stored at −20°C in the dark]. Primary mouse anti-human SIRT1, p53, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and β-actin monoclonal antibodies, and horseradish peroxidase-labeled secondary goat anti-mouse polyclonal antibody (cat. nos. sc-135792, sc-47698, sc-509,sc-20067, sc-58673 and sc-2005, were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). SIRT1, p53 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene primer sequences were synthesized by Nanjing KeyGen Biotech Co., Ltd.
The study was approved by the Ethics Committee of the Second Hospital of Shandong University (Jinan, China). Informed consents were signed by the patients and/or guardians.
Main methods
Cell culture and grouping
Human bladder cancer T24 cell lines were cultured in high-glucose DMEM containing 10% FBS. After meeting the experimental conditions, cells in the logarithmic growth phase were taken for experiments. After centrifugation at 860 × g for 5 min at 4°C, cell sediment was taken, and cells were resuspended and inoculated into culture plates in different specifications according to the experimental requirements. After cells fully adhered to the wall, puerarin in a given concentration (0–200 µmol/l), SIRT1 agonist SRT1720 (1 µmol/l) or SIRT1 inhibitor EX527 (1 µmol/l) was added for routine culture under 5% CO2 at 37°C for a predetermined time, followed by subsequent experiment.
Detection of T24-cell proliferation via MTT assay
Experimental subjects were divided into blank control, control and experimental groups. Human bladder cancer T24 cells were inoculated into a 96-well plate, and the cell density was controlled at 5×103 cells/well, and six repeated wells were set in each group. After cells adhered to the wall, 1% bovine serum albumin containing no puerarin (control group) and containing 20, 50, 100 and 200 µmol/l puerarin (experimental groups) were added, respectively, for incubation in an incubator with 5% CO2 at 37°C for 24, 48 and 72 h. Then, 200 µl DMSO was added into each well and vibrated at a low speed on a shaking table for 10 min to completely dissolve the crystals. The protocol was repeated 3 times. The absorbance value of each well was measured at a wavelength of 490 nm by using a microplate reader (Bio-Rad 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The blank control group (without cells and puerarin) was used for zero setting. Cell inhibition rate = 1 - (absorbance valueexperimental group - absorbance valueblank control group)/(absorbance valuecontrol group - absorbance valueblank control group) ×100%.
Detection of T24-cell apoptosis via flow cytometry
T24 cells in the logarithmic growth phase were inoculated into the petri dish, and treated with puerarin (100 µmol/l), SRT1720 (1 µmol/l) and EX527 (1 µmol/l) for 24 h according to the experimental requirements. After digestion with trypsin, adherent cells were collected, counted and washed with phosphate-buffered saline (PBS) twice. Then, the cells in each group were resuspended in 500 µl binding buffer, and 5 µl Annexin V and 5 µl propidium iodide were added, followed by incubation in the dark at room temperature for 15 min. Finally, the apoptosis rate was detected via flow cytometry (Thermo Fisher Scientific, Inc.).
Detection of protein expression levels via western blotting
T24 cells in the logarithmic growth phase were inoculated into the petri dish, and treated with puerarin (100 µmol/l), SRT1720 (1 µmol/l) and EX527 (1 µmol/l) for 24 h according to the experimental requirements. Cells were added with the radioimmunoprecipitation assay (RIPA) lysis buffer at 4°C and incubated for 30 min. The total protein was extracted, and the protein concentration was detected by using the bicinchoninic acid (BCA) kit, and the protein curve was drawn. Then, 60 µg total protein was taken for sample spotting, and isolated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), after which the protein was transferred onto a polyvinylidene fluoride (PVDF) membrane under 100 V for 150 min, blocked with 5% skim milk powder at room temperature for 1 h, and incubated with primary antibody (1:500) at 4°C overnight, with β-actin (1:600) as the internal reference. After being washed with PBS, the protein was added with the secondary antibody (1:800) for incubation at 37°C for 2 h. Finally, the band image was collected after electrochemiluminescence development (Bio-Rad Laboratories, Inc.) and the optical density value of protein band was measured by using the gel imaging analysis system (Bio-Rad Laboratories, Inc.). The gray value of the target protein band was scanned by Quantity One software, and the expression intensity of each group was indicated by the ratio of the gray value of the target protein band to β-actin band.
Detection of messenger ribonucleic acid (mRNA) expression via reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
T24 cells in the logarithmic growth phase were taken and treated with puerarin (100 µmol/l), SRT1720 (1 µmol/l) and EX527 (1 µmol/l) for 24 h according to the experimental requirements. RNA was extracted from cells by using TRIzol reagent via one-step method, reverse transcribed into complementary deoxyribonucleic acid (cDNA) and then amplified into DNA in strict accordance with the manufacturers instructions of the reverse transcription and SYBR-Green I real-time fluorescence quantitative polymerase chain reaction (PCR) kit, followed by quantitative analysis of the target gene. Primer sequences of SIRT1: forward, 5′-CTGCCTGGATCCCCTTAGTTTTG-3′ and reverse, 5′-GGGCCTGTTGCTCTCCTCATTAA-3′. Primer sequences of p53: forward, 5′-CCGGCGCACAGAGGAAGAGA-3′ and reverse, 5′-TGGGGAGAGGAGCTGGTGTTGT-3′. Primer sequences of GAPDH as an internal reference: forward, 5′-CATGGGGTGTGAACCATGAGA-3′ and reverse, 5′-GTCTTCTGGGTGGCAGTGAT-3′. The 2−ΔΔCq method was used for quantification (10). The thermocycling conditions were as follows: Predenaturation at 95°C for 10 min, then denaturation at 95°C for 30 sec, annealing at 50°C for 30 sec, at 95°C, extension at 70°C for 1 min, 40 cycles, and finally extending 10 min at 70 °C. The experiment was repeated 3 times.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 22.0 software (IBM Corp., Armonk, NY, USA) was used for data entry, and data were collected and statistically analyzed. t-test was used for the comparison of measurement data between the two groups, and one-way analysis of variance was used for the data comparison among groups and the post hoc test was the Least Significant Difference test. P<0.05 indicated that the difference was statistically significant.
Results
Inhibitory effect of puerarin on proliferation of bladder cancer T24 cells (Fig. 1)
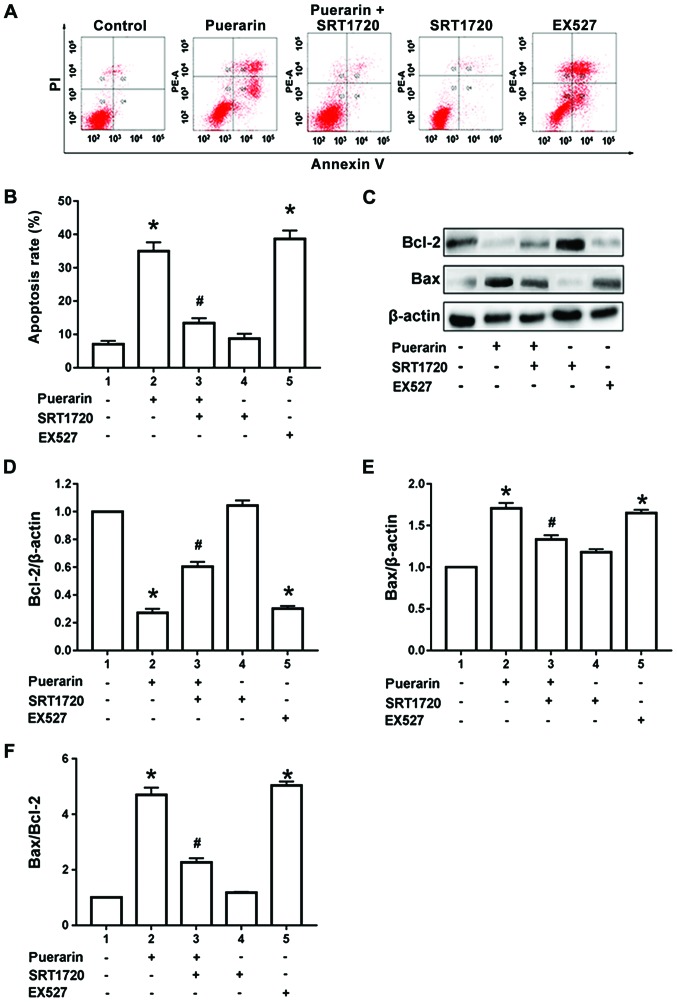
Figure 1.
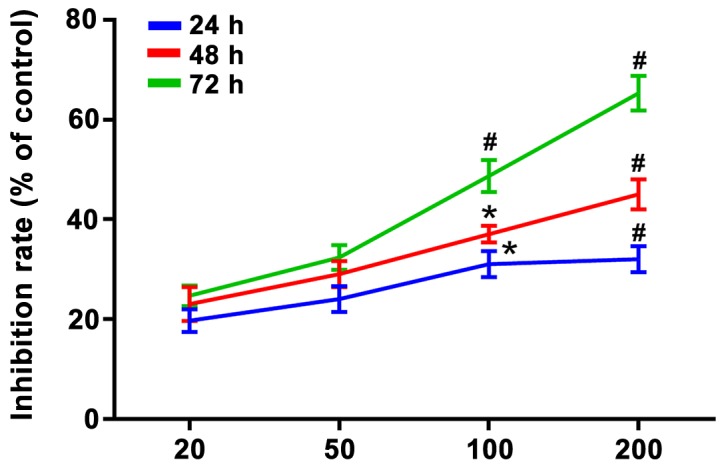
Inhibitory effect of puerarin on proliferation of bladder cancer T24 cells. The cell inhibition rates under 100 and 200 µg/ml puerarin at 24, 48 and 72 h were higher compared with those in the normal control group (p<0.05). *P<0.05 and #P<0.01 vs. the normal control group.
Results of MTT assay revealed that the inhibition rate of bladder cancer T24 cells was significantly increased after treatment with puerarin at different concentrations (0, 20, 50, 100 and 200 µg/ml), and it was elevated with the increase of treatment time and dose, displaying an obvious dose-effect relationship. A total of 20 and 50 µg/ml puerarin could inhibit the proliferation of T24 cells, and there was no statistically significant difference compared with the normal control group (p>0.05). The cell inhibition rates under 100 µg/ml puerarin at 24, 48 and 72 h had statistically significant differences compared with those in the normal control group (p<0.05). Besides, there were also statistically significant differences in the cell inhibition rates under 200 µg/ml puerarin at 24, 48 and 72 h compared with those in the normal control group (p<0.01).
Puerarin induces apoptosis of bladder cancer T24 cells
Results of flow cytometry manifested that compared with control group, 100 µg/ml puerarin could significantly increase the proportion of apoptosis in bladder cancer T24 cells (p<0.05). However, compared with that in the puerarin group, the proportion of apoptotic T24 cells was significantly decreased after pre-treatment with SIRT1 agonist SRT1720 for 1 h and then treatment with 100 µg/ml puerarin for 24 h (p<0.05). Compared with the control group, the administration of SRT1720 alone did not increase the proportion of apoptotic T24 cells, and the difference was not statistically significant (p>0.05). However, the administration of SIRT1 inhibitor EX527 alone for 24 h could simulate the effect of puerarin to induce T24-cell apoptosis (p<0.05) (Fig. 2A and B). According to the results of western blotting, compared with the control group, 100 µg/ml puerarin could obviously increase the Bax protein expression and decrease the Bcl-2 protein expression in T24 cells, so the Bax/Bcl-2 ratio was increased significantly (p<0.05). Moreover, compared with those in the puerarin group, expression of the Bax and Bcl-2 protein in T24 cells were reversed, and the Bax/Bcl-2 ratio was decreased after administration of SIRT1 agonist SRT1720 (p<0.05). Compared with those in the control group, the Bax protein expression was not increased and the Bcl-2 protein expression was not decreased after administration of SRT1720 alone, and there were no statistically significant differences (p>0.05). However, the administration of SIRT1 inhibitor EX527 alone for 24 h could simulate the effect of puerarin to increase the Bax protein expression and Bax/Bcl-2 ratio and decrease the Bcl-2 protein expression (p<0.05) (Fig. 2C-F).
Figure 2.
Puerarin induces apoptosis of bladder cancer T24 cells. (A and B) Flow cytometry was conducted to determine apoptosis of T24 cells treated with puerarin, SRT1720, EX527 and puerarin combined with SRT1720. (C-E) The protein levels of Bax, Bcl-2 were determined by western blotting in T24 cells treated with puerarin, SRT1720, EX527 and puerarin combined with SRT1720. (F) The Bax/Bcl-2 ratio in T24 cells treated with puerarin, SRT1720, EX527 and puerarin combined with SRT1720. *P<0.05 vs. control group, #P<0.01 vs. puerarin group. Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2.
Effect of puerarin on SIRT1/p53 pathway in bladder cancer T24 cells
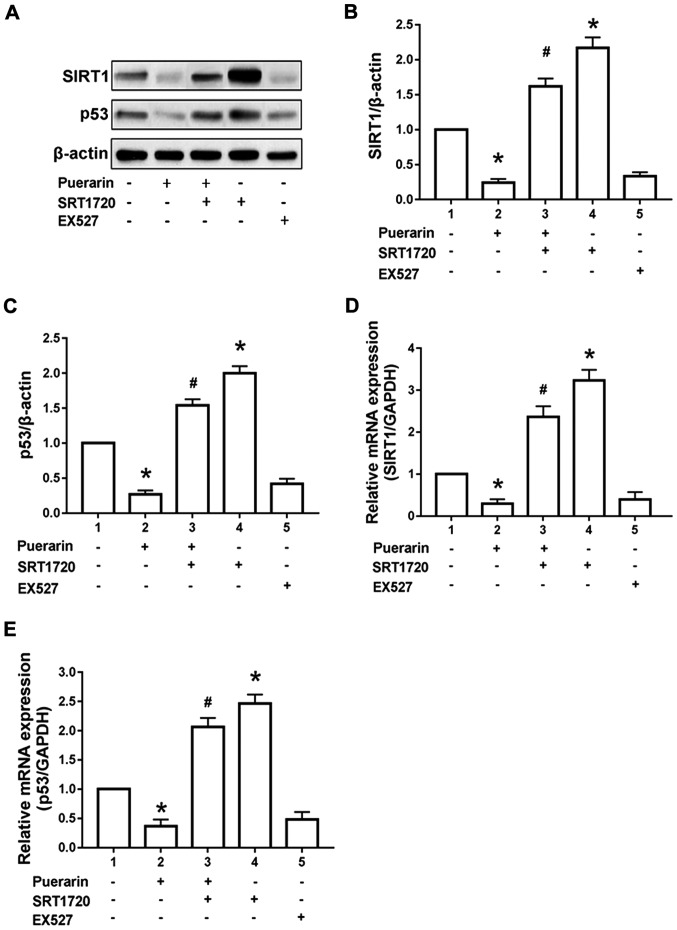
Results of western blotting manifested that compared with control group, 100 µg/ml puerarin could remarkably reduce expression of SIRT1 and p53 protein in T24 cells (p<0.05). Compared with those in the puerarin group, expression of SIRT1 and p53 protein in T24 cells could be reversed after administration of SIRT1 agonist SRT1720. The admini-stration of SRT1720 alone could increase the SIRT1 and p53 protein expression compared with the control group (p<0.05). However, the administration of SIRT1 inhibitor EX5272 alone for 24 h simulated the effect of puerarin to reduce the SIRT1 and p53 protein in T24 cells (p<0.05) (Fig. 3A-C). According to the results of RT-qPCR, 100 µg/ml puerarin could obviously reduce the expression of SIRT1 and p53 mRNA in T24 cells compared with the control group (p<0.05). Compared with those in the puerarin group, the expression of SIRT1 and p53 mRNA in T24 cells was reversed after administration of SIRT1 agonist SRT1720. The administration of SRT1720 alone could increase the SIRT1 and p53 mRNA expression compared with the control group (p<0.05). However, the administration of SIRT1 inhibitor EX5272 alone for 24 h could simulate the effect of puerarin to reduce the expression of SIRT1 and p53 mRNA in T24 cells (p<0.05) (Fig. 3D and E).
Figure 3.
Effect of puerarin on SIRT1/p53 pathway in bladder cancer T24 cells. (A-C) The protein levels of SIRT1, and p53 were determined by western blotting in T24 cells treated with puerarin, SRT1720, EX527 and puerarin combined with SRT1720. (D and E) The mRNA expression of SIRT1 and p53 in T24 cells treated with puerarin, SRT1720, EX527 and puerarin combined with SRT1720. *P<0.05 vs. control group, #P<0.01 vs. puerarin group. SIRT1, silent information regulator 1; mRNA, messenger ribonucleic acid.
Discussion
Bladder cancer is one of the frequently occurring tumors in men, as well as an important cause of cancer death in men. Studies have demonstrated that heavy smoking and drinking will significantly increase the onset risk of bladder cancer, which are not conducive to the prognosis and improvement of life quality of patients (11). However, due to the high recurrence and metastasis rates of bladder cancer, its clinical treatment is often restricted, and there is still lack of reliable and effective treatment means. Therefore, it is of great importance to find a new treatment method.
There is considerable research showing that traditional Chinese medicine can play a regulatory role in disease at a macro-level, and possesses excellent curative effects on delaying, controlling and preventing the recurrence of disease (12). Puerarin is an isoflavone substance extracted from plants such as Pueraria thomsonii. Numerous studies have revealed that puerarin significantly inhibits the proliferation of a variety of breast cancer cells in a time- and dose-dependent manner (13,14). Besides, puerarin can induce apoptosis through the classic caspase apoptosis pathway, and arrest cells in the G2/M phase (15). Results of this study manifested that the proliferation capacity of bladder cancer T24 cells remarkably declined after treatment with puerarin at different concentrations (0, 20, 50, 100 and 200 µg/ml) in a time- and dose-dependent manner, which were consistent with the above views.
Blocked apoptosis is one of the causes of the infinite proliferation of tumor cells. Bcl-2 family genes and p53 genes are all important in regulating apoptosis. Bcl-2 and Bax, as the two most representative genes in the Bcl-2 family, exert effects of inhibiting and promoting apoptosis, respectively. When Bcl-2 is relatively predominant in cells, Bcl-2 will form a heterodimer with Bax, thus inhibiting apoptosis. However, when Bax is predominant in cells, Bax itself will form the homodimer, thus promoting apoptosis (16,17). Therefore, the Bax/Bcl-2 ratio has important significance for the occurrence and degree of apoptosis. In this study, it was found that 100 µg/ml puerarin could significantly inhibit the proliferation of bladder cancer T24cells, increase the Bax protein expression, and reduce the Bcl-2 protein expression, so the Bax/Bcl-2 ratio was obviously increased, thereby inducing apoptosis. After administration of SIRT1 agonist SRT1720, the expression of Bax and Bcl-2 protein in T24 cells was reversed, and the Bax/Bcl-2 ratio was decreased. Besides, the administration of SIRT1 inhibitor EX5272 alone for 24 h simulated the effect of puerarin to increase the Bax protein expression and the Bax/Bcl-2 ratio, and decrease the Bcl-2 protein expression, suggesting that puerarin can be used as a novel drug in the treatment of bladder cancer. However, the specific mechanism of puerarin in inhibiting proliferation and inducing apoptosis of bladder cancer cells has not been clarified yet, and it is pending further study.
In recent years, the major role of SIRT1 gene in the occurrence and development of tumors has attracted extensive attention. SIRT1 is an important member of the nicotinamide (NAD+)-dependent class III histone deacetylase sirtuin family (18). Previous studies found that SIRT1 plays a crucial role in regulating the senescence and substance metabolism of tumor cells (19). Some studies have manifested that the low expression of SIRT1 can induce apoptosis of ovarian cancer cells (20,21), but little is known about SIRT1 in bladder cancer cells. In addition, studies have shown that SIRT1 can downregulate the p53 expression through inhibiting acetylation of p53 protein, thus promoting cell growth (22). In this study, it was found that SIRT1 and p53 protein and mRNA expression in bladder cancer T24 cells could be obviously reduced by 100 µg/ml puerarin, but they could be significantly increased after pre-treatment of T24 cells with SRT1720 for 1 h. Administration of EX527 alone in the treatment of T24 cells could stimulate the effect of puerarin to reduce expression of SIRT1 and p53 protein and mRNA. Therefore, puerarin may induce apoptosis of bladder cancer cells via inhibiting SIRT1/p53 pathway, but its specific regulatory mechanism remains unclear.
In conclusion, this experiment suggested that puerarin can inhibit proliferation and induce apoptosis of bladder cancer T24 cells, and proved that SIRT1/p53 signaling pathway is involved in the pathological process of apoptosis of human bladder cancer T24 cells, laying a foundation for the application of puerarin in prevention and treatment of bladder cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
GY designed the study and drafted the manuscript. SK collected and analyzed the data. JC performed RT-qPCR. XL was responsible for western blotting. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Second Hospital of Shandong University (Jinan, China). Informed consents were signed by the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mowlds DS, Foster CE, Ichii H. Invasive squamous cell bladder cancer of the ureterovesical junction in a renal transplant patient: A case report. J Surg Case Rep. 2017;2017:rjx066. doi: 10.1093/jscr/rjx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood DP. Re: Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of Bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS) J Urol. 2014;191:1731. doi: 10.1016/j.juro.2014.03.073. [DOI] [PubMed] [Google Scholar]
- 3.Zahoor H, Elson P, Stephenson A, Haber GP, Kaouk J, Fergany A, Lee B, Koshkin V, Ornstein M, Gilligan T, et al. Patient characteristics, treatment patterns and prognostic factors in squamous cell bladder cancer. Clin Genitourin Cancer. 2018;16:e437–e442. doi: 10.1016/j.clgc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Arruda RP, Mariano MB, Pereira CF, Lima GC, Lessa TN, Neto MC. Laparoscopic cystoprostatectomy for bladder cancer in a male patient combined with open ileal conduit urinary diversion. Int Braz J Urol. 2017;43:169–170. doi: 10.1590/s1677-5538.ibju.2014.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang K, Chen H, Tang K, Guan W, Zhou H, Guo X, Chen Z, Ye Z, Xu H. Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15:167–174. doi: 10.3892/ol.2017.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang PP, Zhu XF, Yang L, Liang H, Feng SW, Zhang RH. Puerarin stimulates osteoblasts differentiation and bone formation through estrogen receptor, p38 MAPK, and Wnt/β-catenin pathways. J Asian Nat Prod Res. 2012;14:897–905. doi: 10.1080/10286020.2012.702757. [DOI] [PubMed] [Google Scholar]
- 7.Guo XF, Yang ZR, Wang J, Lei XF, Lv XG, Dong WG. Synergistic antitumor effect of puerarin combined with 5-fluorouracil on gastric carcinoma. Mol Med Rep. 2015;11:2562–2568. doi: 10.3892/mmr.2014.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin MH, Lee YH, Cheng HL, Chen HY, Jhuang FH, Chueh PJ. Capsaicin inhibits multiple bladder cancer cell phenotypes by inhibiting tumor-associated NADH oxidase (tNOX) and sirtuin1 (SIRT1) Molecules. 2016;21:849. doi: 10.3390/molecules21070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Reulen RC, de Vogel S, Zhong W, Zhong Z, Xie LP, Hu Z, Deng Y, Yang K, Liang Y, Zeng X, et al. Physical activity and risk of prostate and bladder cancer in China: The South and East China case-control study on prostate and bladder cancer. PLoS One. 2017;12:e0178613. doi: 10.1371/journal.pone.0178613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang JL, Liu BY, Ma KW. Traditional Chinese medicine. Lancet. 2008;372:1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Chen H, Wang Y, Zhang J. In vitro and in vivo antitumour activities of puerarin 6″-O-xyloside on human lung carcinoma A549 cell line via the induction of the mitochondria-mediated apoptosis pathway. Pharm Biol. 2016;54:1793–1799. doi: 10.3109/13880209.2015.1127980. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage of NF-κB and Erk pathway. Biomed Pharmacother. 2017;92:429–436. doi: 10.1016/j.biopha.2017.05.102. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z, Li W. Induction of apoptosis by puerarin in colon cancer HT-29 cells. Cancer Lett. 2006;238:53–60. doi: 10.1016/j.canlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Al-Qathama A, Gibbons S, Prieto JM. Differential modulation of Bax/Bcl-2 ratio and onset of caspase-3/7 activation induced by derivatives of justicidin B in human melanoma cells A375. Oncotarget. 2017;8:95999–96012. doi: 10.18632/oncotarget.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Qin F, Yang L, Xian J, Zou Q, Jin H, Wang L, Zhang L. Nucleophosmin mutations induce chemosensitivity in THP-1 leukemia cells by suppressing NF-κB activity and regulating Bax/Bcl-2 expression. J Cancer. 2016;7:2270–2279. doi: 10.7150/jca.16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan L, Wang L, Wang X, He M, Yao X. SIRT1 contributes to neuroendocrine differentiation of prostate cancer. Oncotarget. 2017;9:2002–2016. doi: 10.18632/oncotarget.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YY, Sun FL, Zhang Y, Wang Z. SIRT1 acts as a potential tumor suppressor in oral squamous cell carcinoma. J Chin Med Assoc. 2018;81:416–422. doi: 10.1016/j.jcma.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Shuang T, Wang M, Zhou Y, Shi C. Over-expression of Sirt1 contributes to chemoresistance and indicates poor prognosis in serous epithelial ovarian cancer (EOC) Med Oncol. 2015;32:260. doi: 10.1007/s12032-015-0706-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Chen J, Sun L, Xu Y. SIRT1 deacetylates KLF4 to activate Claudin-5 transcription in ovarian cancer cells. J Cell Biochem. 2018;119:2418–2426. doi: 10.1002/jcb.26404. [DOI] [PubMed] [Google Scholar]
- 22.Han L, Liang XH, Chen LX, Bao SM, Yan ZQ. SIRT1 is highly expressed in brain metastasis tissues of non-small cell lung cancer (NSCLC) and in positive regulation of NSCLC cell migration. Int J Clin Exp Pathol. 2013;6:2357–2365. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.