Abstract
Microorganisms can be found almost anywhere, including in and on the human body. The collection of microorganisms associated with a certain location is called a microbiota, with its collective genetic material referred to as the microbiome. The largest population of microorganisms on the human body resides in the gastrointestinal tract; thus, it is not surprising that the most investigated human microbiome is the human gut microbiome. On average, the gut hosts microbes from more than 60 genera and contains more cells than the human body. The human gut microbiome has been shown to influence many aspects of host health, including more recently the brain.
Several modes of interaction between the gut and the brain have been discovered, including via the synthesis of metabolites and neurotransmitters, activation of the vagus nerve, and activation of the immune system. A growing body of work is implicating the microbiome in a variety of psychological processes and neuropsychiatric disorders. These include mood and anxiety disorders, neurodevelopmental disorders such as autism spectrum disorder and schizophrenia, and even neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases. Moreover, it is probable that most psychotropic medications have an impact on the microbiome.
Here, an overview will be provided for the bidirectional role of the microbiome in brain health, age-associated cognitive decline, and neurological and psychiatric disorders. Furthermore, a primer on the common microbiological and bioinformatics techniques used to interrogate the microbiome will be provided. This review is meant to equip the reader with a primer to this exciting research area that is permeating all areas of biological psychiatry research.
Keywords: microbiome, microbiology techniques, gut-brain-axis
Introduction
When the Dutchman Antonie van Leeuwenhoek peered through his homemade microscope in the seventeenth century, he dubbed the kleine diertjens (tiny animals) he found there animalcules (Lane, 2015). The discovery that microorganisms are residing practically everywhere, including in and on humans, had a profound impact on medical knowledge. A short time later, the link between these small, bloodless animals and a diarrhea epidemic was suggested by Valk (Valk, 1745). In 1890, Robert Koch published his famous postulates in an attempt to formulate criteria that would establish whether a given microbe causes a given disease (Koch, 1876). Up until recently in medicine, we have regarded microorganisms as undesirable germs to be kept at bay. They were thought to range from pathogenic to harmless to humans and relevant to almost all areas of medicine.
Nonetheless, the disciplines of microbiology and psychiatry evolved along distinct trajectories with only a few notable exceptions. Infamously, the psychiatrist Henry Cotton had the teeth of psychiatric patients in his care removed, believing microbes on their teeth to be the source of their illness (Anderson et al., 2017). There is also a report in the British Journal of Psychiatry in 1910 of the successful treatment of melancholia with Lactic acid bacillus (Phillips, 1910). An early adopter of the idea of microorganisms as beneficial was the 1908 winner of the Nobel Prize in Physiology and Medicine, Metchnikoff. He was convinced of the beneficial effects of fermented milk for “autointoxication” (a rather broad term encompassing a range of negative health outcomes, including fatigue and melancholia; Bested et al., 2013), so much so that it has been reported that he drank fermented milk daily. Despite Metchnikoff’s early hypotheses regarding the potential health benefits of certain bacterial strains, these ideas were largely ignored for the better part of a century.
However, in the last decade, developments in sequencing technology and bioinformatics have allowed in-depth investigations into the composition of complex microbial ecosystems as well as the metabolic and metagenomic potential of such systems. Ventures like MetaHIT (Qin et al., 2010), the Human Microbiome Project (Methé et al., 2012), the ELDERMET study (Claesson et al., 2012), the Belgian Flemish Gut Flora Project (Falony et al., 2016), and the Dutch LifeLines-DEEP (Tigchelaar et al., 2015) have shed light on the bidirectional relationship between microorganisms and their hosts. This marks a pivotal change in our view of microbes. Not only do we now view microorganisms as a cause of disease, they are also increasingly seen as a cause of health (Bloomfield et al., 2016).
The largest population of microorganisms on the human body resides in the gastrointestinal tract. Known as the gut microbiota, this complex ecosystem is comprised of microorganisms including bacteria, fungi, and archaea from over 60 genera (Falony et al., 2016). Recent estimates put the total bacterial count on an average human at around 3.0 × 1013, which is just more than the estimates of human cells in the body (Sender et al., 2016). In a 70-kg individual, the human gut microbiota would weigh in at an impressive 0.2 kg (Sender et al., 2016). The total genetic material of this mass is known as the microbiome. In terms of genes we are more than 99% microbial, meaning the vast majority of both genes and DNA found in a human originates from microbes (Qin et al., 2010). Perhaps the most surprising development to arise from this field has been the realization that the microbiome plays a key role in the programming of all major body systems, including the brain (Round and Mazmanian, 2009; Diaz Heijtz et al., 2011; Collins et al., 2012; Cryan and Dinan, 2012; Foster et al., 2016; Kundu et al., 2017).
In this review, an overview of our knowledge on the microbiome in relation to the brain will be provided. First, the development and function of the microbiome will be discussed in the context of health and well-being. Next, we will provide the reader with a summary of tools used to investigate the microbiome. Finally, the focus is brought to evidence for the role of the microbiome in states of stress and disease, including psychiatric disorders, age-associated cognitive decline, and neurological disorders. To aid the reader, a glossary is included (see Box 1). The overall goal of this review is to highlight the need to further study and better understand the microbiome, particularly with respect to its role in psychiatric health and disease. Not only does the microbiome represent a tremendously valuable therapeutic target for numerous psychiatric illnesses, but it is also a promising target for the general improvement of physical, mental, and cognitive states in healthy individuals.
Box 1. Glossary of microbiome-associated terms
| Term | Definition |
|---|---|
| 16S rRNA gene/transcript sequencing | Bioinformatics technique where highly conserved regions of the 16S rRNA gene (DNA) or transcript (cDNA) are used to identify present or metabolically active microbes in a sample, respectively. |
| Alpha diversity, beta diversity, gamma diversity | Statistical terms used in ecology to describe variability of a dataset. Alpha diversity describes within-sample variability, while beta-diversity describes variability between samples. Gamma diversity is rarely used and describes variability between all samples in the dataset. Many different formulas are available that define diversity differently, putting different weights on aspects like the number of species, how rare/abundant the species are, binary presence/abundance, and even taxonomic distance between species. |
| Enterotype | Name for a controversial type of microbiome classification based on the proportions of certain microbes. |
| Fecal microbiota transplantation (FMT) | Treatment where subjects are colonized with processed fecal matter (usually from a healthy donor in clinical cases or from a specific clinical population of interest in experimental studies). To ensure grafting of the donor microbiome, antibiotics (or germ-free animals) are generally used to deplete the recipient microbiome prior to FMT. |
| Flux balance analysis (FBA) | Computational technique used to predict the metabolic behavior of an organism. In other words, what metabolic pathways will be more or less active in an organism in a given environment. |
| Germ-free (GF) | A host without a microbiome. Generally refers to mice and rats that were born and reared in a sterile environment to keep them from developing a microbiome, for the purpose of experimentation. |
| GreenGenes, SILVA, RDP | Sequence databases and tools used to identify which microbes are present in a sample and their taxonomic relationships. |
| Holobiont | Term describing the collection of a host and its microbiomes. |
| Host | The organism (e.g., human, rodent etc.) that houses a given microbiome population. |
| Microbiome | A term often used synonymously with “microbiota” but more precisely used to refer to the collective genome of a given microbiota. |
| Microbiota | The collection of microorganisms found in/on a particular environment or living host. |
| PICRUSt, HUMAnN2, LEfSe, GraPhlAn, MetaPhlAn | Parts of the bioBakery set, software tools developed by the Huttenhower lab, used to analyze microbiome data. (https://bitbucket.org/biobakery/biobakery/wiki/Home) |
| Prebiotics | Nondigestible foods (such as fibers) that have a beneficial effect on the microbiome for the host. |
| Principal coordinate analysis (PCoA) | Statistical method used for datasets with many numerical values per sample, like microbiota data. The complex data are algorithmically converted to simpler set of values, called principal coordinates, with the aim of explaining variation in the data. Useful for visualizing differences between microbiome samples. If a principal coordinate is large, this is an indication it is determining a large proportion of the observed variance in the data. |
| Probiotics | Live microbes that have a positive effect on host health when ingested in adequate quantities. |
| Psychobiotics | Targeted interventions of the microbiome to support mental or brain health. |
| QIIME, QIIME2 | Quantitative Insights Into Microbial Ecology: Software tools used to analyze microbiome data. |
| Phylum->Class->Order->Family->Genus->Species->Strain | Increasingly granular taxonomic levels used to classify lifeforms. Frequently used in the microbiome field. |
| Synbiotics | Synergistic combination of prebiotics and probiotics. The aim is to optimize treatment effects by providing both the beneficial microbes and the nutrients they need to survive and colonize. |
| Whole genome shotgun sequencing | Bioinformatics technique where all DNA in a sample is sequenced to identify which microbes are present in a sample and their functional (metagenomic) potential. More expensive than 16S rRNA sequencing, but gives more reliable functional predictions. |
The “Healthy” Microbiome
It is worth reminding ourselves that we are living in a microbial world; microbes were here first and there has never been a time when the brain existed without microbes (Stilling et al., 2014). It makes sense to consider the human host in the context of its environment. While scientific reductionism is a powerful tool, a more holistic systems biology approach has enabled us to more accurately understand complex interactions (Sugihara et al., 2012). In this spirit, the term holobiont, describing the totality of the host and its microorganisms, has gained increasing traction in the field (Bordenstein and Theis, 2015; Theis et al., 2016). By blurring the borders between otherwise clearly defined organ systems, the holobiont provides a useful concept for understanding the many levels of interaction between the host and its microbiome.
Where It Began
The composition of the microbiome is not only unique to each individual but is also known to differ drastically throughout the host’s lifespan. For the most part, colonization of the human gut microbiome is thought to begin at birth, although this notion has become subject to debate based on recent reports of microbial DNA in the placenta and meconium (Stout et al., 2013; Aagaard et al., 2014). While these reports remain controversial (Perez-Muñoz et al., 2017), what is clear is that the neonate is exposed to the vaginal microbiome of the mother during delivery through the birth canal. In contrast, when the newborn is delivered via Caesarean section (C-section), it is exposed to the skin microbiome rather than the vaginal microbiome (Chu et al., 2017). Consequently, the microbiome of children delivered via C-section differs significantly from that of children delivered vaginally (Dominguez-Bello et al., 2010; Dominguez-Bello et al., 2016). Other factors, such as prematurity, breastfeeding, the presence of pets, parental smoking, maternal age, weight (especially obesity), and race are also known to impact the developing microbiome (Borre et al., 2014; Bokulich et al., 2016; Levin et al., 2016).
They Are What You Eat
As the infant develops, it seems that some of these early factors become less influential. For example, the microbiota of infants born by C-section or natural delivery converges over time, becoming indistinguishable by 6 weeks of age (Chu et al., 2017; Hill et al., 2017). However, one factor that continues to have a significant impact on microbiota composition throughout the lifespan is the diet of the host (David et al., 2014; Sandhu et al., 2017). In particular, the research shows a stark contrast between the Western diet, with its high sugar, animal fat, and carbohydrate content, in comparison to a Mediterranean diet, which is characterized by increased variety of foods and higher fiber content. The microbiota profile of individuals with these different diets is drastically different (Wu et al., 2011; De Filippis et al., 2016). Although previous studies have segregated different mammalian gut microbiomes based on their compositions, known as enterotypes, this concept has been challenged and is still in the process of being refined (Costea et al., 2018). While there is still debate over the canonical number of enterotypes in humans, there is a general consensus that a division can be made between an enterotype enriched at the genus level in Prevotella and one enriched in Bacteroides. Strikingly, this difference can be related to dietary intake. Specifically, fiber-rich diets are associated with the Prevotella enterotype, reflecting the role of Prevotella species in production of hydrolases specialized for plant fiber degradation (Purushe et al., 2010). Bacteroides, on the other hand, are associated with the Western diet (Costea et al., 2018).
The Microbiota-Gut-Brain Axis
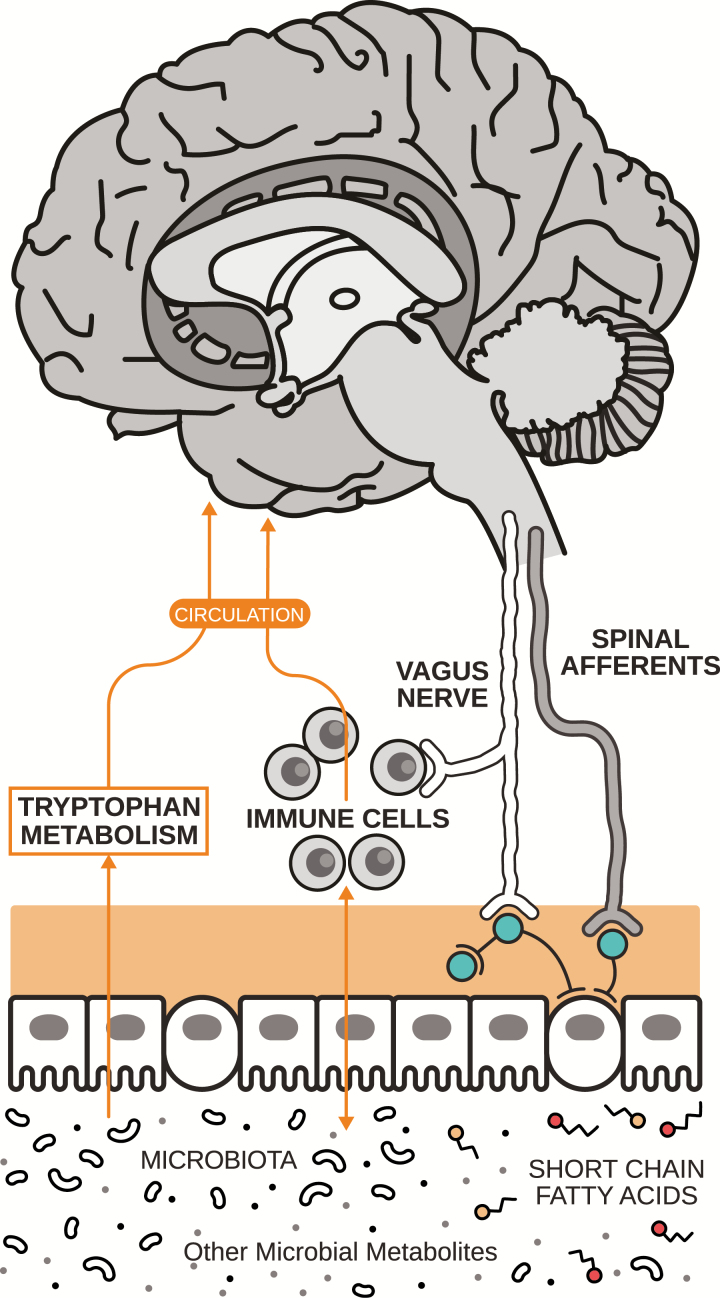
The gut microbiota is known to interact with the brain indirectly, in a bidirectional manner, most likely through a variety of pathways including vagal nerve stimulation, interaction with the immune system, and microbial production of human neurotransmitters (see Figure 1; Cryan and Dinan, 2012; Lyte, 2014; Yano et al., 2015; Schirmer et al., 2016; Kennedy et al., 2017). While the precise mechanisms of action remain unknown, evidence for bidirectional communication between the microbiome and the brain is clear and the impact striking.
Figure 1.
The gut-brain axis. Pathways of communication between the gut microbiome and the brain include vagal nerve stimulation, interaction with short-chain fatty acids, immunoregulatory elements, and tryptophan metabolism. In addition, certain microbes are known to produce and secrete human neurotransmitters. Figure adapted from Cowan et al. (2018).
Besides regulating brain function, the microbiome has also been shown to regulate the physical development of the brain (Dinan and Cryan, 2017b). For instance, hypermyelination of prefrontal cortex neurons has been observed in the brains of germ-free mice (Hoban et al., 2016). Moreover, the dendrites of neurons in the amygdala and hippocampus of germ-free mice are morphologically distinct from those in control mice (Luczynski et al., 2016b). In a recent study, mouse pups born from germ-free mothers were colonized with microbiota from either slow- or fast-growing human infants (Lu et al., 2018). Pups with microbiota from fast-growing infants showed an accelerated neuronal differentiation when compared with slow-growing humanized and germ-free pups. In addition, slow-growing humanized mice were found to exhibit more signs of neuroinflammation. Finally, the microbiota-derived molecule Pglyrp2, which was determined to cross the blood-brain barrier, has been shown to influence the protein expression profile in the germ-free mouse model (Arentsen et al., 2017).
Completing the circle, not only does targeting the gut microbiome influence the brain, there is research that suggests targeting the brain also influences the gut microbiome. There have been several recent studies indicating that certain pharmaceuticals, especially psychotropic agents, can shape the microbiome (Davey et al., 2012, 2013; Kao et al., 2018; Maier et al., 2018). The best evidence for psychotropic effects on the microbiota have been observed with antipsychotic drugs (Davey et al., 2012, 2013; Kao et al., 2018). In addition, most classes of antidepressants, including the widely used selective serotonin receptor inhibitors, have also been shown to impact the microbiota, exhibiting antimicrobial activity in vitro (Munoz-Bellido et al., 2000; Macedo et al., 2017). These findings are suggestive of a potential whole microbiota-gut-brain axis effect of certain psychotropics, consistent with the effects of stress and psychological state on this axis (Cryan and Dinan, 2012; Moloney et al., 2014; Foster et al., 2017). However, it is difficult to disentangle whether such effects are mediated by changes in signaling from the brain to the gut microbiota or, alternatively, via direct actions of the drugs on the microbiota. Other tools and models such as brain stimulation and traumatic brain injury are now being used to establish brain-to-microbiota influences more directly. Brain stimulation research is still very much in the preliminary stages; only one conference abstract on this topic has been published, which reported that deep transcranial magnetic stimulation improves symptoms of obesity by modulating gut microbiota (Ferrulli et al., 2018). In a controlled experimental model of stroke in mice, changes in the cecal microbiota were observed within 72 hours after brain damage was induced (Houlden et al., 2016). This work replicates clinical findings from a patient population of Chinese stroke victims who exhibited altered microbiota composition compared with asymptomatic controls (Yin et al., 2015). Together, these studies highlight the substantial influence of the brain over the microbiota, which we are only just beginning to understand.
Tools to Interrogate the Microbiome
Over the years, a plethora of different experimental models have been utilized to investigate the microbiome and its interactions with the host. Here, some of the most common will be discussed. For the most part, mice and rats are used as hosts when modelling the microbiome. While both animals have distinct features compared with humans, there are many similarities and advantages, making them the preferred models in most studies (Nguyen et al., 2015). However, many other species from drosophila (Leitão-Gonçalves et al., 2017) to zebrafish (Borrelli et al., 2016) and up to primates (Bailey and Coe, 1999; McKenney et al., 2015; Amaral et al., 2017) have also been used to investigate the microbiome. As the field of microbiota-brain interactions matures, we can expect that more studies will be carried out in healthy humans and clinical populations, which will further strengthen the conclusions that can be drawn from this line of research.
Microbiota Depletion: Germ-Free Animals and Antibiotics
As in all aspects of science and engineering, one of the main ways to confirm the importance of a specific process is to remove it and study the consequences. Germ-free animals represent our best available model for complete removal of all microorganisms. This method has been instrumental in linking the microbiome to many key brain processes and behaviors (Diaz Heijtz et al., 2011; Luczynski et al., 2016a, 2016b). However, given that germ-free animals exhibit such dramatically abnormal neurodevelopment, it is difficult to determine the precise role of the microbiome in said processes (Al-Asmakh and Zadjali, 2015; Luczynski et al., 2016a). Moreover, this is an extreme model with limited clinical translation.
While at first glance similar to the germ-free model, antibiotics represent an alternative distinct model to investigate the microbiome (Lundberg et al., 2016). Antibiotics have the advantage that they can be used to knock out/down the microbiota for specified timeframes without affecting neurodevelopmental programming per se. However, as antibiotic treatments can negatively impact the animals’ health, it is sometimes hard to distinguish the side-effects of the antibiotics from the microbiome-driven effects (Luczynski et al., 2016a). Moreover, many antibiotics can cross the blood brain barrier (Nau et al., 2010), and caution is therefore required when interpreting studies of antibiotic-induced microbiota depletion.
“Friends with Benefits”: Prebiotics, Probiotics, Synbiotics, and Psychobiotics
While disruption of the microbiome can have a negative effect on the host, supplementing the microbiome has been used as a strategy to optimize host performance. Introducing probiotic microbes that are known or suspected to be beneficial is an intuitive way to investigate the relationship between the host and the microbiome. Here, it is important to note that it is likely not just specific microbes that may be beneficial, but the collateral effects of that strain on the microbial ecosystem in given niches (Duran-Pinedo and Frias-Lopez, 2015). Although the term probiotic has gained substantial public attention and become part of the wider vocabulary, it is important to clarify that many commercially available strains marketed as probiotics have never been tested in clinical trials and therefore by definition would not meet the criteria of conferring a health benefit.
Prebiotics represent a more general way to alter microbiome composition, essentially providing nutrients to encourage the growth of beneficial microorganisms (Gibson et al., 2017). However, prebiotics are considered less specific than probiotics, as there is little control over which microorganisms will metabolize the prebiotics and which will proliferate. A growing body of work is now focused on combining prebiotics and probiotics to develop synbiotics (Ford et al., 2014). Finally, and most recently, the term psychobiotics has been introduced to describe targeted microbiome interventions with a beneficial effect on mental health, which is of particular interest to the study of psychiatric disorders (Dinan et al., 2013; Sarkar et al., 2016; Anderson et al., 2017). Overall, these approaches are appealing because they can be introduced in food and drink and therefore provide a relatively noninvasive method of manipulating the microbiota. While these studies show the potential of probiotics, negative studies have demonstrated that similar probiotic treatments can vary in effectiveness, suggesting that there are more factors at play than just the specific probiotic strain used (Hojsak et al., 2015, Mazurak et al., 2015). This conforms with the understanding that the behavior of a microbial strain is dependent on its metabolic, microbial, and host environment (Succurro et al., 2018).
Fecal Microbiota Transplantation (FMT)
The concept of fecal microbiome transplantation (FMT) as a therapeutic intervention is disrupting Western medicine completely. The procedure involves introducing fecal microbiota from a selected donor to the gastrointestinal tract of the recipient, with the aim of making the recipient microbiome more similar to that of the donor (Borody and Khoruts, 2011). When used as a therapeutic intervention, donors must be screened to ensure they are healthy, as phenotypes like obesity and depression have been shown to be transferable via FMT, at least in rodents (Turnbaugh et al., 2006; Kelly et al., 2016). FMT used in a preclinical setting can involve deliberately unhealthy donor phenotypes. The realization that patients with recurrent Clostridium difficile infection have a good chance to recover after FMT treatment represents an arguably noninvasive and cheap approach to an otherwise difficult to treat disease (Gianotti and Moss, 2017). Moreover, the potential of FMT as a clinical and experimental tool is reflected in the application of this approach to treat a wide variety of diseases (e.g., irritable bowel syndrome, steatohepatitis, ulcerative colitis, and even autism (Pinn et al., 2015; Ren et al., 2015; Kang et al., 2017; Zhou et al., 2017) and investigations of the effects of inter-species FMT from specific clinical populations to experimental rodents (Arrieta et al., 2016). Intriguingly, FMT from young donors to middle-aged recipients has even been used to extend the lifespan of killifish (Smith et al., 2017).
Cross-Sectional Studies
One of the most widely used methods to study the microbiome in humans is to assess microbiome composition across cohorts of clinical patients and matched controls. Thanks to the increasing number of such studies including the microbiome in their measurements, there are a large number of databases available for interrogation, such as the Human Pan Microbial Communities Database (Forster et al., 2016) and the NIH Human Microbiome Project (The N. I. H. H. M. P. Working Group et al., 2009). Here, it is important to note that it is often problematic to pool measurements from different databases together because the exact techniques used for extraction and processing of microbial genetic material account for a large part of the variation between samples (Clooney et al., 2016).
Analysis of the Microbiome
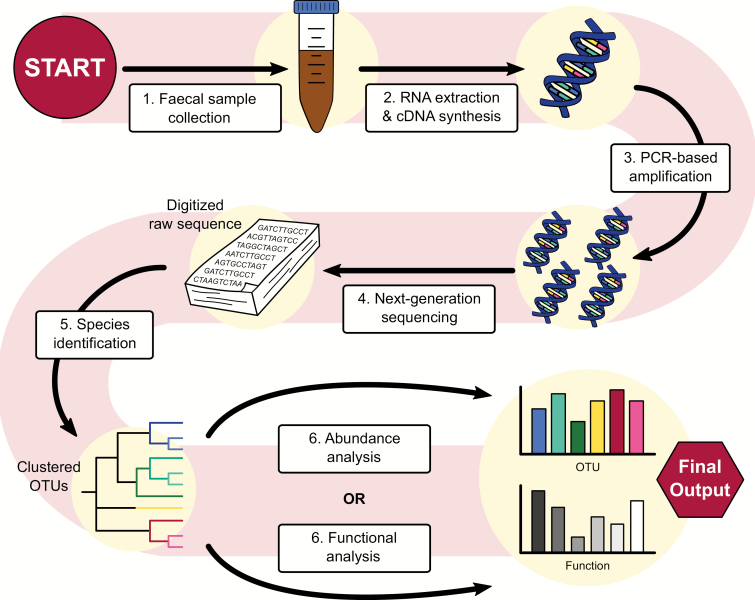
The field of bioinformatics is rapidly developing in response to the large amount of big microbiome datasets released thanks to Next Generation Sequencing (for a review of analysis techniques, see Claesson et al., 2017). Both 16S rRNA gene sequencing and whole genome shotgun sequencing techniques allow for analysis of compositional analysis and functional analysis of the microbiome (see Figure 2). It should be noted that because of the highly dynamic nature of the field, it is difficult to establish standard data-analysis protocols. Given the impact of both sequencing and in silico methods on the outcomes of analysis (Voigt et al., 2015; Clooney et al., 2016), we stress the importance of precise documentation when reporting microbiome research to ensure the reproducibility of findings.
Figure 2.
From stool to statistics. Overview of a sample method used to analyze the gut microbiome using 16S sequencing, a popular technique in microbiome research. Stool samples are collected and, potentially after being stored at -80°C, are prepared for analysis. RNA is extracted from the sample and a cDNA library is generated in preparation for amplification by PCR. Using next-generation sequencing platforms like Illumina and 454 pyrosequencing, the cDNA library is digitalized. From here, species can be identified by clustering the sequences and comparing them with a reference database. Popular databases for this purpose are RDP, SILVA, and, while arguably outdated, GreenGenes. The table of identified taxa can be used for abundance analysis and comparison using metrics like alpha diversity and beta diversity, principal coordinate analysis (PCoA) and differential abundance to quantify differences between samples or groups of samples on platforms like QIIME2. Using this same table, metagenomic data can be inferred, which can be used to make predictions about the functional implications of the observed differences in microbiome composition.
Linking the Microbiome to Psychiatric Disorders
Given the many modes of communication between the brain and the gut microbiome, it is not difficult to imagine the impact the gut microbiome has on host mental health and illness. Here, we first discuss the role of the gut microbiome in stress regulation, as stress is one of the most potent risk factors for psychiatric illness. We then briefly discuss the current state of the evidence linking the microbiome to various psychiatric disorders, from developmental disorders to mood, anxiety, and eating disorders.
The Microbiome and Stress
There is a robust association between stress, which is associated with activation of the hypothalamus-pituitary-adrenal (HPA) axis, and the state of the microbiome (for reviews, see Moloney et al., 2014; Gur et al., 2015; de Weerth, 2017; Foster et al., 2017). A number of studies have demonstrated that stress alters the composition of the microbiota in a range of different hosts, from rats and mice (Gareau et al., 2007; O’Mahony et al., 2011; Golubeva et al., 2015; Bharwani et al., 2016; Burokas et al., 2017) to Syrian hamsters (Partrick et al., 2018), pigs (Mudd et al., 2017), and nonhuman primates (Bailey and Coe, 1999; Bailey et al., 2011).
In the other direction, the gut microbiome also regulates the stress response. In a seminal study, Sudo et al. (2004) elegantly demonstrated that germ-free mice exhibit elevated HPA axis responses to stress as measured by adrenocorticotropic hormone and corticosterone. The HPA axis response was found to be normalized by colonization with a probiotic species but exaggerated by colonization with an enteropathogen in the same study. Similarly, probiotics have been shown to reverse stress effects in many studies using various animal models (Gareau et al., 2007; Desbonnet et al., 2010; Bravo et al., 2011; Ait-Belgnaoui et al., 2012; Barouei et al., 2012; Liang et al., 2015; Cowan et al., 2016; Bharwani et al., 2017; Callaghan et al., 2016). Promisingly, there is analogous evidence that probiotics promote stress resilience or reduce stress-induced physical symptoms and cognitive deficits in humans (Diop et al., 2008; Langkamp-Henken et al., 2015; Kato-Kataoka et al., 2016; Allen et al., 2017; Wang, 2017; Papalini et al., 2018). Finally, certain prebiotics have also been shown to protect against stress-induced effects on the microbiome, physiology, and behavior (Tarr et al., 2015; Burokas et al., 2017).
Attention Deficit Hyperactivity Disorder (ADHD)
Diet has long been considered a potential predisposing factor for attention deficit hyperactivity disorder (ADHD; Jacobson and Schardt, 1999; Pelsser et al., 2011), and many studies testing the effects of elimination diets on symptoms of ADHD have produced positive outcomes (for reviews, see Jacobson and Schardt, 1999; Millichap and Yee, 2012). Of note, a Western-style diet is associated with increased risk for ADHD (Howard et al., 2011). Given the known influence of diet on the microbiome, it has been proposed that the association between diet and ADHD may be driven by the microbiome. Providing preliminary support for this hypothesis, altered microbiome composition has been observed in a clinical cohort of adolescents and adults with ADHD (Aarts et al., 2017). Using predictive functional analysis, it was found that these differences were likely to lead to differential regulation/synthesis of dopamine precursors, changes that were associated with decreased reward anticipation. Finally, one small longitudinal randomized control study of a perinatal probiotic intervention found that probiotic-treated children were less likely to be diagnosed with ADHD (Pärtty et al., 2015).
Autism Spectrum Disorder (ASD)
There is a strong relationship between autism spectrum disorders (ASD) and gastrointestinal disorders, including high rates of comorbidity between these seemingly disparate diagnoses (Hsiao, 2014) and evidence of a correlation between the severity of ASD and severity of gastrointestinal complaints (Adams et al., 2011). When examining the microbiome, several studies have observed differences in children diagnosed with ASD, including relatively low representation of members of the Prevotella genus and other fermenting microbes compared with typically developing children (Kang et al., 2013). While these correlative studies do not provide evidence of causality (Mayer et al., 2014), the case for investigating microbiome-based treatments for ASD continues to strengthen. One recent small-scale pilot study of FMT for ASD showed promising results (Kang et al., 2017), while perinatal probiotic treatment reduced the risk for ASD (Pärtty et al., 2015). Moreover, preclinical studies demonstrate the crucial role of the microbiota in many mouse models of autism (e.g., maternal immune activation, maternal high-fat diet, and the BTBR genetic model; Hsiao et al., 2013; Buffington et al., 2016; Golubeva et al., 2017) as well as specific symptoms of ASD such as abnormal social behavior (Desbonnet et al., 2014).
Schizophrenia
Recently, there have been calls to investigate the link between schizophrenia and the microbiome (Dinan et al., 2014; Severance et al., 2015). As in ASD, there are high rates of gastrointestinal problems reported in schizophrenia (Severance et al., 2015). This finding may be related to the proposed immune origins of the disorder (Patterson, 2009; van Kesteren et al., 2017) and provides a theoretical foundation for investigating the microbiome in schizophrenia, given the key role the microbiome plays in establishing and maintaining immune function (Hooper et al., 2012; Belkaid and Hand, 2014). A recent preliminary study of patients with first-episode psychosis identified differences in the microbiota composition, including reduced prevalence of Lactobacillus and Bifidobacteria species compared with healthy age-matched controls (Schwarz et al., 2018). Importantly, differences in the microbiota were correlated with severity of negative symptoms and risk for remission at 12-month follow-up but did not correlate with duration of antipsychotic drug treatment. Other studies have identified differences in the composition and functional potential of the oropharyngeal microbiome of individuals diagnosed with schizophrenia (Yolken and Dickerson, 2014; Castro-Nallar et al., 2015). Although probiotics have been proposed as a potential adjunctive treatment for schizophrenia, only one published study has examined the efficacy of this approach. Dickerson et al. (2014) found no effects of probiotic treatment on positive or negative symptoms, although the chosen probiotic (Lactobacillus rhamnosus GG and Bifidobacterium animalis subs. lactis) reduced the risk for severe bowel problems in a small group of outpatients with moderate to severe schizophrenia symptoms.
Bipolar Disorder
It has been proposed that microbiome-mediated immune activation may contribute to the onset of bipolar disorder (Dickerson et al., 2017). This hypothesis seems to have originated from the observation that patients with bipolar mania were approximately twice as likely as other patients to have been recently treated with systemic antibiotics (Yolken et al., 2016). Since then, evidence implicating the microbiota in bipolar disorder has started to build. The microbiome of bipolar patients has been found to differ from healthy controls, at least for patients with more severe symptoms (Evans et al., 2017). Specifically, significant differences in 2 separate genera of Firmicutes were observed (one being a reduction in Faecalibacterium, which has also been observed in major depression; see below), with these and several other genera correlating to symptom severity. Moreover, a recent pilot study has shown that probiotic supplementation reduces rates of rehospitalization in patients who have been recently discharged following hospitalization for mania (Dickerson et al., in press).
Major Depression
While the study of the microbiome in schizophrenia and bipolar disorder is still in its infancy, there is stronger (and continually mounting) evidence that the microbiome plays a role in major depression (Foster and McVey Neufeld, 2013; Dash et al., 2015). Germ-free mice display reduced depressive-like behavior; in the forced swim test of behavioral despair, germ-free mice will continue to swim or attempt to escape an inescapable pool for longer than control mice (Zheng et al., 2016), and both probiotic and prebiotic treatments have been shown to reduce depressive-like behavior in rodent models (Desbonnet et al., 2010; Bravo et al., 2011; Burokas et al., 2017). These studies seem to hold translational value, with several systematic reviews indicating that probiotics effectively improve mood in humans (Huang et al., 2016; Pirbaglou et al., 2016; Wallace and Milev, 2017). It is worth noting, though, that one such systematic review found that benefits were limited to those with mild to moderate depression (i.e., healthy individuals did not significantly benefit; Ng et al., 2018), which, alongside probiotic strain differences, may explain some of the conflicting findings in the attempts to translate probiotic effects to humans (Allen et al., 2016; Kelly et al., 2017).
Clinically, several studies have found an altered microbial composition in patients with major depression (Naseribafrouei et al., 2014; Jiang et al., 2015; Kelly et al., 2016; Zheng et al., 2016). Of note, 2 studies reported a reduction in the relative abundance of Faecalibacterium (Jiang et al., 2015; Zheng et al., 2016), mirroring the results described earlier for bipolar disorder (Evans et al., 2017). Jiang et al. (2015) went further to identify a negative correlation between the severity of depression and the prevalence of Faecalibacterium. Another study reported lower levels of Bifidobacterium and Lactobacillus in depressed patients (Aizawa et al., 2016). Strikingly, when the gut microbiome of depressed humans has been transferred to either rats or mice via FMT, the recipient animals exhibit greater depressive- and anxiety-like behavior compared with those that received FMT from healthy humans (Kelly et al., 2016; Zheng et al., 2016).
Anxiety Disorders
There is clear preclinical evidence to support a link between anxiety and the microbiome (Foster and McVey Neufeld, 2013; Malan-Muller et al., 2018). Germ-free mice and zebrafish exhibit reduced anxiety-like behavior (Diaz Heijtz et al., 2011; Neufeld et al., 2011; Clarke et al., 2013; Davis et al., 2016), although germ-free rats exhibit more anxiety-like behavior compared to conventionally colonized controls (Crumeyrolle-Arias et al., 2014). Anxiety-associated microbiome differences have also been observed between strains of mice, with the anxious BALB/c having a distinct microbiome profile compared with the more resilient Swiss Webster strain (Bercik et al., 2011). Furthermore, FMT from one mouse strain to the other was sufficient to partially transfer the respective behavioral phenotypes (i.e., BALB/c mice given NIH Swiss microbiota became less anxious, whereas NIH Swiss mice given BALB/c microbiota became more so).
Additional preclinical studies have shown that probiotic and prebiotic treatments can reduce anxiety-like behaviors in rodents (e.g., Bravo et al., 2011, Burokas et al., 2017). Unfortunately, very few studies have examined the relationship between anxiety and the microbiome in clinical populations. A single, small study of a South African population revealed specific phylum-level differences in the microbiome for those diagnosed with posttraumatic stress disorder compared with trauma-exposed controls (Hemmings et al., 2017). Aside from this correlational study, there have been 2 small intervention studies showing that probiotics reduce self-reported anxiety in healthy individuals (Messaoudi et al., 2011) and in a clinical group presenting with chronic fatigue syndrome (Rao et al., 2009).
Obsessive-Compulsive Disorders (OCD)
While there have been no direct investigations (as of yet) into the microbiome in obsessive-compulsive disorder (OCD) patients, several researchers have speculated that there may be a link (Rees, 2014; Turna et al., 2016). This hypothesis is based on 2 lines of observation. First, it has been noted that many of the risk factors for onset of OCD are also known to disrupt the microbiome, including stress, pregnancy, and antibiotic use (Rees, 2014). Second, there is preclinical evidence that OCD-like behavior in rodents (frequently measured using the marble burying test, which aims to assess repetitive, compulsive behaviors, one of the core symptoms of OCD) can be modified by microbial treatments, including germ-free environments and probiotic treatments (Nishino et al., 2013; Kantak et al., 2014; Savignac et al., 2014).
Eating Disorders
Perhaps unsurprisingly, given their highly impoverished nutrient intake, the microbiota composition of patients suffering from anorexia nervosa differs significantly from that of healthy individuals (Kleiman et al., 2015; Morita et al., 2015; Mack et al., 2016). In the first of these studies, by Kleiman et al. (2015), differences in anxiety, depression, and eating disorder psychopathology were all correlated to microbiota composition. Furthermore, microbiota composition changed during treatment and weight gain, moving closer to the composition observed in healthy control groups, although never fully recovering (Kleiman et al., 2015; Mack et al., 2016).
At the other end of the spectrum, the gut microbiome has also been linked to diet-induced obesity (Torres-Fuentes et al., 2017). Obese individuals exhibit differences in microbiota composition (Ley et al., 2005; Turnbaugh et al., 2006, but see also Sze and Schloss, 2016). Importantly, a causal contribution of the microbiome to diet-induced weight gain has been demonstrated using mice with a humanized microbiome (Turnbaugh et al., 2009). In these mice, switching from a plant-based diet to a Western-style diet caused rapid shifts in the microbiome composition (within 24 hours) and subsequent weight gain. Furthermore, the increased adiposity associated with the Western diet could then be transferred to naïve mice via FMT. Offering hope that we might utilize the microbiome to enact positive weight changes as well, it has been hypothesized that the microbiome may contribute to weight loss following bariatric surgery, based on evidence that such surgeries induce microbiome alterations in both humans and rodents (Peat et al., 2015; Torres-Fuentes et al., 2017).
In patients suffering from disorders that are associated with altered eating habits, it will continue to be difficult to disentangle the direction of microbiome-mental health relationships. It is intriguing to consider this problem; is eating behavior “manipulated” by an altered microbiome (as has been suggested by some, e.g., Alcock et al., 2014), does eating behavior drive microbiome changes and thereby alter gut-brain communication, or both? When considering this question, it is worth noting that changes in eating habits are not limited to eating disorders but are observed across a variety of psychiatric disorders (including anxiety, ADHD, ASD, depression; Yannakoulia et al., 2008; Ptacek et al., 2014), while epidemiological studies show that healthy dietary patterns are associated with better mental health (O’Neil et al., 2014). It is therefore an important question that deserves ongoing attention. Regardless of the initial cause of these disruptions, the opportunity to utilize dietary or other microbiome-targeting interventions to improve mental health holds great appeal and scientific potential.
Linking the Microbiome to Cognitive Impairment
The gut microbiome has been found to play a role in cognitive function, both in age-related cognitive decline as well as general cognitive performance. Mice that were treated with antibiotics showed decreased hippocampal neurogenesis (Möhle et al., 2016). Cessation of neurogenesis has been linked to general cognitive impairment and the onset of Alzheimer’s disease (Costa et al., 2015; Hollands et al., 2017). FMT from healthy mice restored neurogenesis in the antibiotic-exposed animals, but only when combined with specifically selected probiotics (Möhle et al., 2016). In patients suffering from hepatic encephalopathy, where the patient exhibits neuropsychiatric abnormalities as a result of liver dysfunction, the presence and concentration of certain microbes was correlated to the severity of cognitive impairment (Bajaj et al., 2011).
Just as development in early life has been found to parallel gut microbiome development, several age-related diseases have been similarly linked to the state of the microbiome in both animals (Scott et al., 2017) and humans (Claesson et al., 2012). In a study in elderly Koreans, administration of Lactobacillus helveticus IDCC3801 improved performance in cognitive fatigue tests (Chung et al., 2014). A decline in microbial diversity is associated with a concomitant increase in microglial activation correlated to brain mass differences in the mouse (Von Bernhardi et al., 2015). This contributes to an age-associated inflammatory response known as inflammaging, which in turn has been associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases (Franceschi et al., 2007). Furthermore, the microbiome has been shown to regulate microglia activation; one study showed that germ-free mouse brains expressed defective microglia, which was partially rescued upon restoration of the microbial community to control levels (Erny et al., 2015; Möhle et al., 2016; Thion et al., 2018).
Linking the Microbiome to Neurological Disorders
Alzheimer’s Desease (AD)
In addition to being involved in the general phenomenon of inflammaging, the gut microbiome has also been found to be involved in specific age-related illnesses. In patients with Alzheimer’s disease (AD), Hill et al. (2014) reported a correlation between colonization of certain pathogenic microbes such as Toxoplasma and Clamydophila pneumoniae and progression of the disease. Furthermore, patients suffering from AD were shown to have a less diverse microbiome with distinct compositional differences compared with the healthy microbiome (Vogt et al., 2017). In the same study, the researchers theorized about the high prevalence of proinflammatory, lipopolysaccharide-producing, gram-negative bacteria such as Bacteroides in AD patients and their role in pathogenesis of the disorder (Cattaneo et al., 2017). Finally, germ-free or antibiotic-treated transgenic AD mouse models fail to develop plaques (Harach et al., 2017; Minter et al., 2017).
Parkinson’s Disease (PD)
There is a growing emphasis on the role of the gut-brain axis in the onset of Parkinson’s disease (PD; Dinan and Cryan, 2017a; Perez-Pardo et al., 2017). A number of studies have shown alterations in the microbiome in PD (Scheperjans et al., 2015; Keshavarzian et al., 2015; Heintz-Buschart et al., 2018; Qian et al., 2018; Sun et al., 2018). When mice were colonized with the microbiota of PD patients via FMT, they developed motor deficits and neuroinflammation, 2 hallmark symptoms of PD (Sampson et al., 2016). Additionally, symptoms improved when the mice were treated with antibiotics. Large-scale investigations using the extensive patient records in Denmark and Sweden have shown that vagotomy (or more specifically truncal vagotomy), which removes one of the major routes for microbiota to brain communication, is protective against PD (Svensson et al., 2015; Liu et al., 2017).
Multiple Sclerosis (MS)
The immune-related neurological disease multiple sclerosis (MS) has been convincingly linked to alterations in the microbiome (Berer et al., 2011; Wang and Kasper, 2014; Tremlett and Waubant, 2018). When the microbiome of patients diagnosed with MS was transferred to mice, the animals began exhibiting autoimmune encephalomyelitis, a main symptom of MS (Berer et al., 2017). Certain specific microbial species, like Akkermansia muciniphila and Acinetobacter calcoaceticus, have been identified that are present at significantly higher levels in patients suffering from MS than in the healthy population (Cekanaviciute et al., 2017). When these strains were introduced to mice, they again exhibited symptoms of autoimmune encephalomyelitis.
Conclusions
There are many ways in which the microbiome is connected to brain health. Oftentimes, it is hard to differentiate where the causative elements lie: in the brain or in the gut or in other systems such as the immune system. Therefore, it is not advisable to regard the two organs as separate systems but rather as a vastly more complex ecosystem of molecules, microbes, and neurons that should be approached with an interdisciplinary modus operandi. In the context of health and disease, the role of the microbiome as an ecological entity should not be ignored. Many afflictions discussed here are accompanied by alterations in the composition, or even stability, of the microbiome. Rather than the effect of individual taxa in a vacuum, their role in maintaining homeostasis within the microbiome deserves more scientific attention. Recently the role of guilds, taxonomically distinct but functionally related microbes that are associated with metabolic roles of the microbiome, has been stressed (Banerjee et al., 2018). Reinforcing the important role of diet in maintenance of the microbiota-gut-brain axis, food intake, especially dietary fiber, plays an important role in stabilizing these guilds (Zhao et al., 2018). Dietary interventions are gaining momentum as a plausible and modifiable target for improving mental health via the microbiome (Jacka, 2017; Jacka et al., 2017).
Considering the numerous illnesses that are impacted by the microbiome, it is hard to argue against further research to establish the precise underlying mechanisms involved. A comprehensive understanding of the mechanisms regulating the gut-brain axis in health and disease would be of tremendous benefit in predicting the efficacy of novel psychobiotics as well as potential off-target effects of traditional psychotropics. Developments in artificial intelligence and modelling seem promising in this regard. Techniques like flux balance analysis have been implemented to predict the growth medium required for 2 given microbiota to co-occur. There are still many challenges ahead of us, like how to handle the massive amounts of data that will be generated from mechanistic studies of the complex microbiome community.
For now, simply considering the role of the microbiome in a given disorder could be hugely beneficial. This would not only encourage us to keep up to date with current developments in microbiome research but will also help us understand and personalize treatments. After all, “just a gut feeling” seems to be more substantial than the saying might imply.
Acknowledgments
Thanks to Kenneth J. O’Riordan for comments on the paper.
This work was supported by Science Foundation Ireland through a Centre Award to the APC Microbiome Institute (grant no. SFI/12/RC/2273).
Statement of Interest
The APC Microbiome Institute has conducted research funded by many pharmaceutical and food companies. T.G.D. and J.F.C. have received research funding from Mead Johnson, Cremo, Suntory Wellness, Nutricia, 4D Pharma, and DuPont.
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J(2014)The placenta harbors a unique microbiome. Sci Transl Med 6:237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, Franke B, van Hijum SAFT, Arias Vasquez A(2017)Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 12:e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA(2011)Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V(2012)Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37:1885–1895. [DOI] [PubMed] [Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H(2016)Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 202:254–257. [DOI] [PubMed] [Google Scholar]
- Al-Asmakh M, Zadjali F(2015)Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol 25:1583–1588. [DOI] [PubMed] [Google Scholar]
- Alcock J, Maley CC, Aktipis CA(2014)Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 36:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G(2016)Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 6:e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG(2017)Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms. Curr Med Res Opin 33:1349–1351. [DOI] [PubMed] [Google Scholar]
- Amaral WZ, Lubach GR, Proctor A, Lyte M, Phillips GJ, Coe CL(2017)Social influences on prevotella and the gut microbiome of young monkeys. Psychosom Med 79:888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SC, Cryan JF, Dinan T(2017)The psychobiotic revolution: mood, food, and the new science of the gut-brain connection. Washington, DC: National Geographic Society. [Google Scholar]
- Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, Forssberg H, Diaz Heijtz R(2017)The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 22:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Walter J, Finlay BB(2016)Human microbiota-associated mice: A model with challenges. Cell Host Microbe 19:575–578. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Coe CL(1999)Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M(2011)Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM(2012)Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 302:G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Schlaeppi K, Heijden MG(2018)Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol. doi: 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- Barouei J, Moussavi M, Hodgson DM(2012)Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One 7:e46051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW(2014)Role of the microbiota in immunity and inflammation. Cell 157:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM(2011)The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609. [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G(2011)Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538–541. [DOI] [PubMed] [Google Scholar]
- Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kumpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H(2017)Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A 114:10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM(2013)Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part I - autointoxication revisited. Gut Pathog 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P(2016)Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63:217–227. [DOI] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P(2017)Oral treatment with lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med 15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell-Smith R, Turner P(2016)Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health 136:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ(2016)Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 8:343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, Theis KR(2015)Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol 13:e1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody TJ, Khoruts A(2011)Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 9:88–96. [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF(2014)Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518. [DOI] [PubMed] [Google Scholar]
- Borrelli L, Aceto S, Agnisola C, De Paolo S, Dipineto L, Stilling RM, Dinan TG, Cryan JF, Menna LF, Fioretti A(2016)Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci Rep 6:30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF(2011)Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M(2016)Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165:1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF(2017)Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 82:472–487. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Cowan CSM, Richardson R(2016)Treating generational stress: Effect of paternal stress on offspring memory and extinction development is rescued by probiotic treatment. Psychol Sci 27:1171–1180. [DOI] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA(2015)Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 3:e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, et al. , INDIA-FBP Group (2017)Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE(2017)Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 114:10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM(2017)Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 23:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Park JI, Jung ES, Choi EK, Chae SW(2014)Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods 10:465–474. [Google Scholar]
- Claesson MJ, et al. (2012)Gut microbiota composition correlates with diet and health in the elderly. Nature 488:178–184. [DOI] [PubMed] [Google Scholar]
- Claesson MJ, Clooney AG, O’Toole PW(2017)A clinician’s guide to microbiome analysis. Nat Rev Gastroenterol Hepatol 14:585–595. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF(2013)The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18:666–673. [DOI] [PubMed] [Google Scholar]
- Clooney AG, Fouhy F, Sleator RD, O’ Driscoll A, Stanton C, Cotter PD, Claesson MJ(2016)Comparing apples and oranges?: next generation sequencing and its impact on microbiome analysis. PLoS One 11:e0148028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P(2012)The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742. [DOI] [PubMed] [Google Scholar]
- Costa V, Lugert S, Jagasia R(2015)Role of adult hippocampal neurogenesis in cognition in physiology and disease: Pharmacological targets and biomarkers. In: Cognitive enhancement (Kantak K, Wettstein J, eds), pp99–155. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- Costea PI, Hildebrand F, Manimozhiyan A, Bäckhed F, Blaser MJ, Bushman FD, De Vos WM, Ehrlich SD, Fraser CM, Hattori M(2018)Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 3:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CS, Callaghan BL, Richardson R(2016)The effects of a probiotic formulation (Lactobacillus rhamnosus and L. Helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl Psychiatry 6:e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Hoban AE, Ventura-Silva AP, Dinan TG, Clarke G, Cryan JF(2018)Gutsy moves: The amygdala as a critical node in microbiota to brain signaling. BioEssays 40:1700172. [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S(2014)Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42:207–217. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG(2012)Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. [DOI] [PubMed] [Google Scholar]
- Dash S, Clarke G, Berk M, Jacka FN(2015)The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 28:1–6. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF(2012)Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 221:155–169. [DOI] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O’Sullivan O, Crispie F, Dinan TG, Cryan JF, O’Mahony SM(2013)Antipsychotics and the gut microbiome: olanzapine-induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry 3:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ(2014)Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DJ, Bryda EC, Gillespie CH, Ericsson AC(2016)Microbial modulation of behavior and stress responses in zebrafish larvae. Behav Brain Res 311:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D(2016)High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65:1812–1821. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF(2014)Microbiota is essential for social development in the mouse. Mol Psychiatry 19:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG(2010)Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170:1179–1188. [DOI] [PubMed] [Google Scholar]
- de Weerth C.(2017)Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev 83:458–471. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S(2011)Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Severance E, Yolken R(2017)The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun 62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, Schweinfurth L, Stallings C, Sweeney K, Goga J, Yolken RH (in press) Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. doi: 10.1111/bdi.12652. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, Goga J, Khushalani S, Yolken RH(2014)Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim Care Companion CNS Disord 16:PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Borre YE, Cryan JF(2014)Genomics of schizophrenia: time to consider the gut microbiome?Mol Psychiatry 19:1252–1257. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF (2017a) Gut feelings on Parkinson’s and depression. Cerebrum:cer-04-17. [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF (2017b) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF(2013)Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74:720–726. [DOI] [PubMed] [Google Scholar]
- Diop L, Guillou S, Durand H(2008)Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res 28:1–5. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R(2010)Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107:11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, Mendez K, Knight R, Clemente JC(2016)Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Frias-Lopez J(2015)Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes Infect 17:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M(2015)Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, McInnis MG(2017)The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res 87:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, et al. (2016)Population-level analysis of gut microbiome variation. Science 352:560–564. [DOI] [PubMed] [Google Scholar]
- Ferrulli A, Toscano M, Adamo M, Terruzzi I, Drago L, Luzi L(2018)Effects of deep transcranial magnetic stimulation (dTMS) on anti-inflammatory gut bacterial species in obesity. Diabetes 67 (Supplement 1). doi: 10.2337/db18-2088-P [DOI] [Google Scholar]
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P(2014)Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 109:1547–1561. [DOI] [PubMed] [Google Scholar]
- Forster SC, Browne HP, Kumar N, Hunt M, Denise H, Mitchell A, Finn RD, Lawley TD(2016)HPMCD: the database of human microbial communities from metagenomic datasets and microbial reference genomes. Nucleic Acids Res 44:D604–D609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA(2013)Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. [DOI] [PubMed] [Google Scholar]
- Foster JA, Lyte M, Meyer E, Cryan JF(2016)Gut microbiota and brain function: An evolving field in neuroscience. Int J Neuropsychopharmacol 19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, Rinaman L, Cryan JF(2017)Stress and the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S(2007)Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH(2007)Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti RJ, Moss AC(2017)Fecal microbiota transplantation: from clostridium difficile to inflammatory bowel disease. Gastroenterol Hepatol (N Y) 13:209–213. [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G(2017)Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. [DOI] [PubMed] [Google Scholar]
- Golubeva AV, Crampton S, Desbonnet L, Edge D, O’Sullivan O, Lomasney KW, Zhdanov AV, Crispie F, Moloney RD, Borre YE, Cotter PD, Hyland NP, O’Halloran KD, Dinan TG, O’Keeffe GW, Cryan JF(2015)Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 60:58–74. [DOI] [PubMed] [Google Scholar]
- Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Pérez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG, Cryan JF(2017)Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur TL, Worly BL, Bailey MT(2015)Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, Bolmont T(2017)Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7:41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P(2018)The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings SMJ, et al. (2017)The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 79:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, O’ Toole PW, Stanton C(2017)Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ(2014)Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Front Aging Neurosci 6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF(2016)Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojsak I, Tokić Pivac V, Močić Pavić A, Pasini AM, Kolaček S(2015)Bifidobacterium animalis subsp. Lactis fails to prevent common infections in hospitalized children: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 101:680–684. [DOI] [PubMed] [Google Scholar]
- Hollands C, Tobin MK, Hsu M, Musaraca K, Yu TS, Mishra R, Kernie SG, Lazarov O(2017)Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol Neurodegener 12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ(2012)Interactions between the microbiota and the immune system. Science 336:1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts IS, Denes A(2016)Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun 57:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AL, Robinson M, Smith GJ, Ambrosini GL, Piek JP, Oddy WH(2011)ADHD is associated with a “Western” dietary pattern in adolescents. J Atten Disord 15:403–411. [DOI] [PubMed] [Google Scholar]
- Hsiao EY.(2014)Gastrointestinal issues in autism spectrum disorder. Harv Rev Psychiatry 22:104–111. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK(2013)Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wang K, Hu J(2016)Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN.(2017)Nutritional psychiatry: where to next?Ebiomedicine 17:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M(2017)A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MF, Schardt D(1999)Diet, ADHD & behavior: a quarter-century review. Washington, DC: Center for Science in the Public Interest. [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B(2015)Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194. [DOI] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R(2013)Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8:e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R(2017)Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak PA, Bobrow DN, Nyby JG(2014)Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (lactobacillus rhamnosus GG). Behav Pharmacol 25:71–79. [DOI] [PubMed] [Google Scholar]
- Kao AC, Spitzer S, Anthony DC, Lennox B, Burnet PWJ(2018)Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K(2016)Fermented milk containing lactobacillus casei strain shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes 7:153–156. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG(2016)Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG(2017)Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 61:50–59. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Cryan JF, Dinan TG, Clarke G(2017)Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112:399–412. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM(2015)Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360. [DOI] [PubMed] [Google Scholar]
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM(2015)The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 77:969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R.(1876)Untersuchungen ueber Bakterien V. Die Aetiologie der Milzbrand-Krankheit, begruendent auf die Entwicklungsgeschichte des Bacillus Anthracis. Beiträge zur Biologie der Pflanzen 2:277–310. [Google Scholar]
- Kundu P, Blacher E, Elinav E, Pettersson S(2017)Our gut microbiome: the evolving inner self. Cell 171:1481–1493. [DOI] [PubMed] [Google Scholar]
- Lane N.(2015)The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos Trans R Soc Lond B Biol Sci 370:20140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkamp-Henken B, Rowe CC, Ford AL, Christman MC, Nieves C Jr, Khouri L, Specht GJ, Girard SA, Spaiser SJ, Dahl WJ(2015)Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. Br J Nutr 113:426–434. [DOI] [PubMed] [Google Scholar]
- Leitão-Gonçalves R, Carvalho-Santos Z, Francisco AP, Fioreze GT, Anjos M, Baltazar C, Elias AP, Itskov PM, Piper MDW, Ribeiro C(2017)Commensal bacteria and essential amino acids control food choice behavior and reproduction. Plos Biol 15:e2000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, Kim H, Zoratti EM, Lukacs NW, Boushey HA, Ownby DR, Lynch SV, Johnson CC(2016)Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep 6:31775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI(2005)Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F(2015)Administration of lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310:561–577. [DOI] [PubMed] [Google Scholar]
- Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K(2017)Vagotomy and parkinson disease: A swedish register-based matched-cohort study. Neurology 88:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, Claud EC(2018)Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci Rep 8:5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF (2016a) Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 19:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF (2016b) Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci 44:2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg R, Toft MF, August B, Hansen AK, Hansen CH(2016)Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes 7:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M.(2014)Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 5:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D, Filho AJMC, Soares de Sousa CN, Quevedo J, Barichello T, Júnior HVN, Freitas de Lucena D(2017)Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord 208:22–32. [DOI] [PubMed] [Google Scholar]
- Mack I, Cuntz U, Grämer C, Niedermaier S, Pohl C, Schwiertz A, Zimmermann K, Zipfel S, Enck P, Penders J(2016)Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 6:26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A(2018)Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SMJ(2018)The gut microbiome and mental health: implications for anxiety- and trauma-related disorders. Omics 22:90–107. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Padua D, Tillisch K(2014)Altered brain-gut axis in autism: comorbidity or causative mechanisms?Bioessays 36:933–939. [DOI] [PubMed] [Google Scholar]
- Mazurak N, Broelz E, Storr M, Enck P(2015)Probiotic therapy of the irritable bowel syndrome: why is the evidence still poor and what can be done about it?J Neurogastroenterol Motil 21:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney EA, Rodrigo A, Yoder AD(2015)Patterns of gut bacterial colonization in three primate species. Plos One 10:e0124618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM(2011)Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 105:755–764. [DOI] [PubMed] [Google Scholar]
- Methé BA, Nelson KE, Pop M, Creasy HH, Giglio MG, Huttenhower C, Gevers D, Petrosino JF, Abubucker S, Badger JH(2012)A framework for human microbiome research. Nature 486:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millichap JG, Yee MM(2012)The diet factor in attention-deficit/hyperactivity disorder. Pediatrics 129:330–337. [DOI] [PubMed] [Google Scholar]
- Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, Jabri B, Chang EB, Tanzi RE, Sisodia SS(2017)Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1δE9 murine model of Alzheimer’s disease. Sci Rep 7:10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA(2016)Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 15:1945–1956. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF(2014)The microbiome: stress, health and disease. Mamm Genome 25:49–74. [DOI] [PubMed] [Google Scholar]
- Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, Sudo N(2015)Gut dysbiosis in patients with anorexia nervosa. Plos One 10:e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN(2017)Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 8:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Bellido JL, Munoz-Criado S, Garcìa-Rodrìguez JA(2000)Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents 14:177–180. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K(2014)Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26:1155–1162. [DOI] [PubMed] [Google Scholar]
- Nau R, Sörgel F, Eiffert H(2010)Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA(2011)Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23:255–264. [DOI] [PubMed] [Google Scholar]
- Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS(2018)A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord 228:13–19. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Vieira-Silva S, Liston A, Raes J(2015)How informative is the mouse for human gut microbiota research?Dis Model Mech 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, Aiba Y, Koga Y, Sudo N(2013)Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil 25:521–528. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Hyland NP, Dinan TG, Cryan JF(2011)Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 214:71–88. [DOI] [PubMed] [Google Scholar]
- O’Neil A, Quirk SE, Housden S, Brennan SL, Williams LJ, Pasco JA, Berk M, Jacka FN(2014)Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health 104:e31–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalini S, Michels F, Kohn N, Wegman J, van Hemert S, Roelofs K, Arias Vasquez A, Aarts E(2018)Stress matters: A double-blind, randomized controlled trial on the effects of a multispecies probiotic on neurocognition. bioRxiv doi: 10.1101/263673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partrick KA, Chassaing B, Beach LQ, McCann KE, Gewirtz AT, Huhman KL(2018)Acute and repeated exposure to social stress reduces gut microbiota diversity in syrian hamsters. Behav Brain Res 345:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E(2015)A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res 77:823–828. [DOI] [PubMed] [Google Scholar]
- Patterson PH.(2009)Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- Peat CM, Kleiman SC, Bulik CM, Carroll IM(2015)The intestinal microbiome in bariatric surgery patients. Eur Eat Disord Rev 23:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelsser LM, Frankena K, Toorman J, Savelkoul HF, Dubois AE, Pereira RR, Haagen TA, Rommelse NN, Buitelaar JK(2011)Effects of a restricted elimination diet on the behaviour of children with attention-deficit hyperactivity disorder (INCA study): a randomised controlled trial. Lancet 377:494–503. [DOI] [PubMed] [Google Scholar]
- Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J(2017)A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pardo P, Kliest T, Dodiya HB, Broersen LM, Garssen J, Keshavarzian A, Kraneveld AD(2017)The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol 817:86–95. [DOI] [PubMed] [Google Scholar]
- Phillips JGP.(1910)The treatment of melancholia by the lactic acid bacillus. J Ment Sci 56:422–430. [Google Scholar]
- Pinn DM, Aroniadis OC, Brandt LJ(2015)Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)?Neurogastroenterol Motil 27:19–29. [DOI] [PubMed] [Google Scholar]
- Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P(2016)Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res 36:889–898. [DOI] [PubMed] [Google Scholar]
- Ptacek R, Kuzelova H, Stefano GB, Raboch J, Sadkova T, Goetz M, Kream RM(2014)Disruptive patterns of eating behaviors and associated lifestyles in males with ADHD. Med Sci Monit 20:608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushe J, Fouts DE, Morrison M, White BA, Mackie RI, Coutinho PM, Henrissat B, Nelson KE, North American Consortium for Rumen Bacteria (2010)Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche. Microb Ecol 60:721–729. [DOI] [PubMed] [Google Scholar]
- Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen SD, Xiao Q(2018)Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202. [DOI] [PubMed] [Google Scholar]
- Qin J, et al. , MetaHIT Consortium (2010)A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC(2009)A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JC.(2014)Obsessive-compulsive disorder and gut microbiota dysregulation. Med Hypotheses 82:163–166. [DOI] [PubMed] [Google Scholar]
- Ren R, Sun G, Yang Y, Peng L, Zhang X, Wang S, Dou Y, Zhang X, Wang Z, Bo X, Liu Q, Li W, Fan N, Ma X(2015)A pilot study of treating ulcerative colitis with fecal microbiota transplantation. Zhonghua Nei Ke Za Zhi 54:411–415. [PubMed] [Google Scholar]
- Round JL, Mazmanian SK(2009)The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK(2016)Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF(2017)Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 179:223–244. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ(2016)Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 39:763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF(2014)Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 26:1615–1627. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P(2015)Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358. [DOI] [PubMed] [Google Scholar]
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Horst RT, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A, Fu J, Joosten LAB, Zhernakova A, Huttenhower C, Wijmenga C, Netea MG, Xavier RJ(2016)Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167:1897. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J(2018)Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res 192:398–403. [DOI] [PubMed] [Google Scholar]
- Scott KA, Ida M, Peterson VL, Prenderville JA, Moloney GM, Izumo T, Murphy K, Murphy A, Ross RP, Stanton C, Dinan TG, Cryan JF(2017)Revisiting metchnikoff: age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav Immun 65:20–32. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R(2016)Revised estimates for the number of human and bacteria cells in the body. Plos Biol 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, Yolken RH(2015)Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep 17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR(2017)Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife 6:e27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Bordenstein SR, Dinan TG, Cryan JF(2014)Friends with social benefits: host-microbe interactions as a driver of brain evolution and development?Front Cell Infect Microbiol 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU(2013)Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 208:226.e1–226.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succurro A, Segre D, Ebenhöh O(2018)Emergent sub-population behavior uncovered with a community dynamic metabolic model of Escherichia coli diauxic growth. bioRxiv doi: 10.1101/291492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y(2004)Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara G, May R, Ye H, Hsieh CH, Deyle E, Fogarty M, Munch S(2012)Detecting causality in complex ecosystems. Science 338:496–500. [DOI] [PubMed] [Google Scholar]
- Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ (2018) Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70:48–60. [DOI] [PubMed] [Google Scholar]
- Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT(2015)Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529. [DOI] [PubMed] [Google Scholar]
- Sze MA, Schloss PD(2016)Looking for a signal in the noise: Revisiting obesity and the microbiome. mBio 7:e01018–e01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT(2015)The prebiotics 3’sialyllactose and 6’sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun 50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The N. I. H. H. M. P. Working Group , Peterson J, et al. (2009)The NIH human microbiome project. Genome Res 19:2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, Bosch TCG, Cryan JF, Gilbert SF, Goodnight CJ, Lloyd EA(2016)Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems 1:e00028–e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, et al. (2018)Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172:500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar EF, Zhernakova A, Dekens JA, Hermes G, Baranska A, Mujagic Z, Swertz MA, Muñoz AM, Deelen P, Cénit MC, Franke L, Scholtens S, Stolk RP, Wijmenga C, Feskens EJ(2015)Cohort profile: lifelines DEEP, a prospective, general population cohort study in the northern netherlands: study design and baseline characteristics. BMJ Open 5:e006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF(2017)The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol 2:747–756. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Waubant E(2018)Gut microbiome and pediatric multiple sclerosis. Mult Scler 24:64–68. [DOI] [PubMed] [Google Scholar]
- Turna J, Grosman Kaplan K, Anglin R, Van Ameringen M(2016)“What’s bugging the gut in OCD?” A review of the gut microbiome in obsessive-compulsive disorder. Depress Anxiety 33:171–178. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI(2006)An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI(2009)The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk E.(1745)Geneeskundig verhaal van de algemeene loop-ziekte, die te Kampen, en in de om-geleegene streeken heeft gewoed in ‘t jaar 1736 neevens een werktuigkunstige, en natuurkundige beschryvinge van de oorzaak, uitwerking en genezinge. Haarlem, the Netherlands: Izaak van der Vinne. [Google Scholar]
- van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE(2017)Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7:e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE(2017)Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AY, Costea PI, Kultima JR, Li SS, Zeller G, Sunagawa S, Bork P(2015)Temporal and technical variability of human gut metagenomes. Genome Biol 16:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernhardi R, Eugenín-von Bernhardi L, Eugenín J(2015)Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJK, Milev R(2017)The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.(2017)Effects of probiotics on central nervous system functions in humans. Tübingen, Germany: University of Tübingen. [Google Scholar]
- Wang Y, Kasper LH(2014)The role of microbiome in central nervous system disorders. Brain Behav Immun 38:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD(2011)Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannakoulia M, Panagiotakos DB, Pitsavos C, Tsetsekou E, Fappa E, Papageorgiou C, Stefanadis C(2008)Eating habits in relations to anxiety symptoms among apparently healthy adults. A pattern analysis from the ATTICA study. Appetite 51:519–525. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY(2015)Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L(2015)Dysbiosis of gut microbiota with reduced trimethylamine‐N‐oxide level in patients with large‐artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 4:e002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, Schweinfurth L, Stallings C, Sweeney K, Dickerson F(2016)Individuals hospitalized with acute mania have increased exposure to antimicrobial medications. Bipolar Disord 18:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R, Dickerson F(2014)The microbiome-The missing link in the pathogenesis of schizophrenia. J Schizophr Res 153:S16. [Google Scholar]
- Zhao L, et al. (2018)Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359:1151–1156. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P(2016)Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. [DOI] [PubMed] [Google Scholar]
- Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG(2017)Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 7:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]